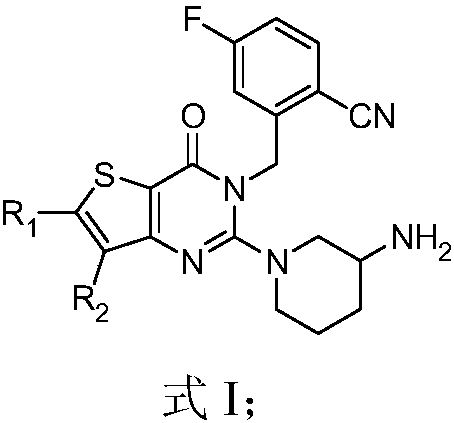
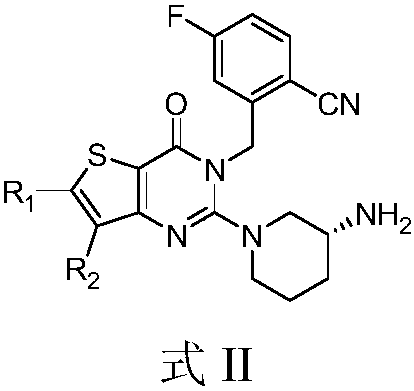
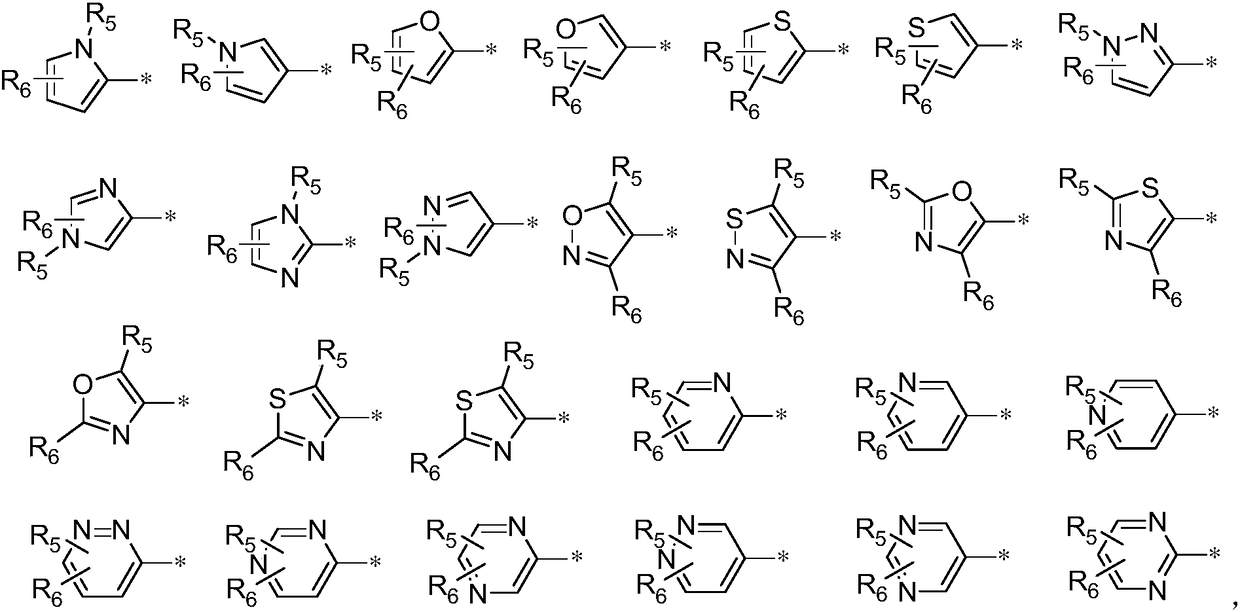
Thienopyrimidinone compound or pharmaceutically-acceptable salt thereof and preparation method and application thereof
A technology for pyrimidone and compound, which is applied in the field of thienopyrimidinone compounds and their preparation, can solve the problems such as the inability to maintain the inhibitory effect for a long time, the control effect is not good, the half-life is not long, and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Example 1 Synthesis of compound 1
[0128] synthetic route:
[0129]
[0130] Synthesis of compound 1-1:
[0131] Add urea (60g, 1mol) into a 250mL dry single-necked round-bottomed flask, heat to 160°C under an oil bath to melt, add 3-aminothiophene-2-carboxylic acid methyl ester (20g, 0.13mol), and the mixture is heated at 190- Heat and react at 200°C for 3 hours, cool, add 500mL of 10% aqueous sodium hydroxide solution, stir evenly, filter with suction, wash with 5-10% aqueous sodium hydroxide solution, and adjust the pH of the filtrate to 7.0 with 2N HCl solution in an ice bath. A white solid was precipitated, suction filtered, washed with ice water, and dried to obtain 12.5 g of a white solid, with a yield of 57%;
[0132] 1 H-NMR (400MHz, d 6 -DMSO): δ6.9 (1H, d, J = 5.2Hz), 8.10 (1H, d, J = 5.2Hz), 11.60-11.1 (2H, br, s); MS: 169.1 [M+H + ].
[0133] Synthesis of Compound 1-2:
[0134] Mix the compound 1-1 (12.5g, 74.3mmol) obtained in the above step wi...
Embodiment 2
[0145] Example 2 .Synthesis of Compound 2
[0146] synthetic route:
[0147]
[0148] Synthesis of Compound 2-1:
[0149] Dissolve methyl 3-amino-2-thiophenecarboxylate (20.4g, 0.13mol) in 300mL THF, add TEA (14.1g, 0.14mol), add trichloroacetyl chloride (25.5g, 0.14mol) dropwise at 2°C, gradually Raise to room temperature and stir for 30 minutes, add 300 mL of water to quench, extract with equal volume of EA three times, combine organic layers, wash with 5% aqueous sodium bicarbonate solution, water and saturated saline successively, dry over anhydrous sodium sulfate, filter, and filtrate The solvent was removed by pressure evaporation to obtain 33.4g compound 2-1, yield 85%; MS: 303.9[M+H + ].
[0150] Synthesis of compound 2-2:
[0151] Dissolve the compound 2-1 (33.4g, 0.11mol) obtained in the above step in 300mL of acetic acid, add liquid bromine (53.0g, 0.33mol) dropwise at 10°C, keep stirring at this temperature for 30 minutes, then heat and stir at 70°C Overn...
Embodiment 3
[0163] Example 3 Synthesis of compound 3
[0164] synthetic route:
[0165]
[0166] Replace the raw material 3-aminothiophene-2-methyl carboxylate in Example 1 with the raw material 3-amino-4-methylthiophene-2-methyl carboxylate, and refer to the synthetic method of Example 1 to synthesize compound 3-1 to compound 2.
[0167] Compound 3-1: 1H-NMR (400MHz, DMSO-d): δ2.20(1H, s), 7.68(1H, s), 11.20(1H, s), 11.38(1H, s); MS: 183.0[M+H + ].
[0168] Compound 3-2: MS: 218.9 [M+H + ].
[0169] Compound 3-3: 1 H-NMR (400MHz, CDCl 3 ):δ2.40(1H,s),7.52(1H,s); MS:200.9[M+H + ].
[0170] Compound 3-4: 1 H-NMR (400MHz, CDCl 3 ): δ2.41(1H,s),5.75(1H,s),6.86(1H,dd,J=2.0Hz,2.0Hz),7.12(1H,m),7.53(1H,s),7.75(1H ,m); MS:334.0[M+H + ].
[0171] Compound 3:MS:398.1[M+H + ].
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com