Continuous synthetic method for 2-fluorophenylboronic acid compound
A technology of fluorophenylboronic acid and compounds, which is applied in the field of continuous synthesis of 2-fluorophenylboronic acid compounds, can solve the problems of high cost and low yield, and achieve low cost, high yield, and improved purity and yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] As described in the background art, the existing methods for preparing 2-fluorophenylboronic acid compounds have the problems of high cost and low yield. In order to solve the above-mentioned technical problems, the application provides a continuous synthesis method of 2-fluorobenzene boronic acid compounds, the continuous synthesis method comprises: the fluorobenzene compound, the first organic solvent and the alkyl lithium reagent in the first The continuous lithiation reaction is carried out in the continuous reaction device to obtain the lithiated product; the continuous borated reaction is carried out on the lithiated product and the alkyl borate in the second continuous reaction device to obtain 2-fluorophenylboronic acid compounds.
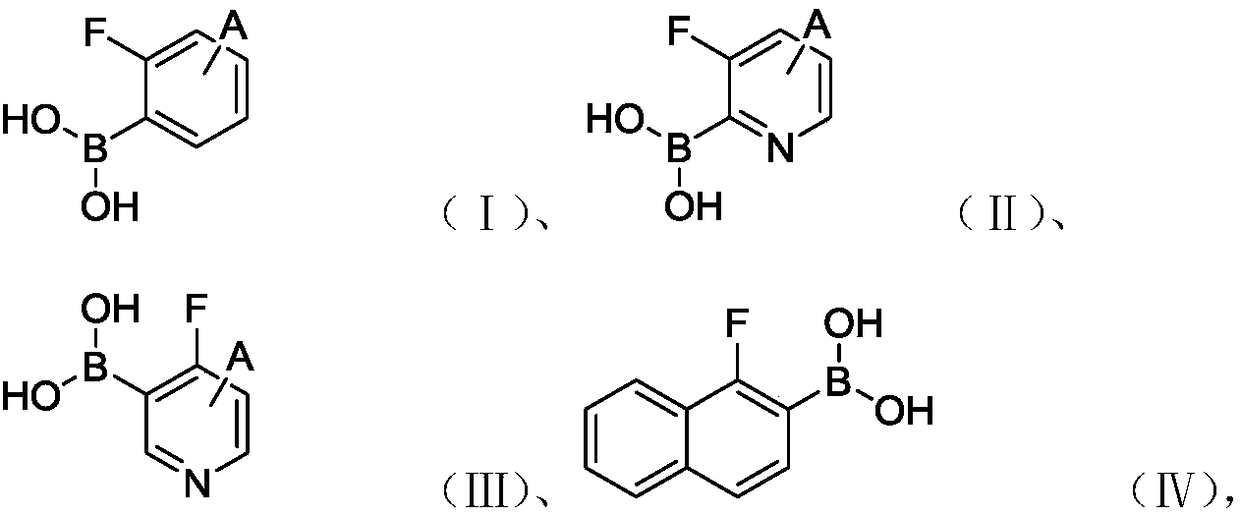
[0039] 2-fluorophenylboronic acid compounds include one or more of the compounds shown in formulas (I) to (IV):
[0040]
[0041] Among them, A includes but not limited to -H, -F, -Cl, methyl, ethyl, isopropyl, methoxy, ethoxy, is...
Embodiment 1
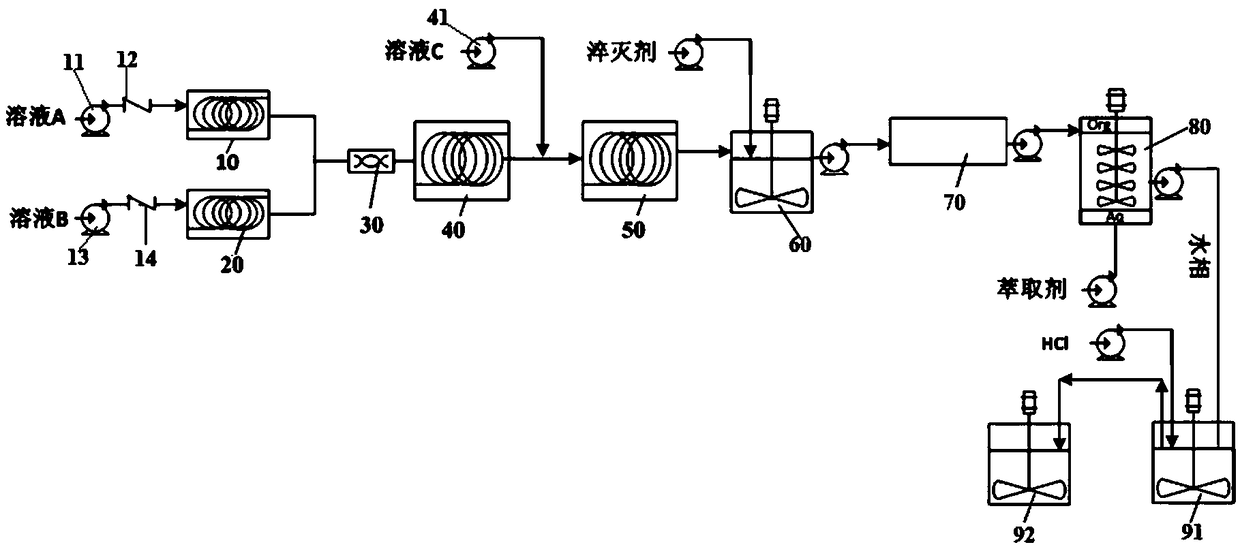
[0083] Dissolve 2-fluorophenylboronic acid (1 equivalent) in the first organic solvent (tetrahydrofuran, 8 mL / g), then add the basic ligand PMDTA (pentamethyldiethylenetriamine, 1.1 equivalent) and mix well to form solution A. n-Butyllithium (22.5 wt% n-hexane solution, 1.6 equivalents) was used as solution B without any dilution. As solution C, isopropyl borate (2.0 equiv) was diluted with THF (2 mL / g).
[0084] according to figure 1 As shown, the continuous reaction equipment is installed and debugged. Solution A, solution B and solution C are sequentially connected to the first material pump 11, the second material pump 13 and the third material pump 41, and the flow rate is automatically controlled by the automatic feeding system. The delivery pipeline of solution A has a first one-way valve 12, and the delivery pipeline of solution B has a second one-way valve 14 (to prevent backflow, solution C decides whether to install a one-way valve as required).
[0085] According...
Embodiment 2
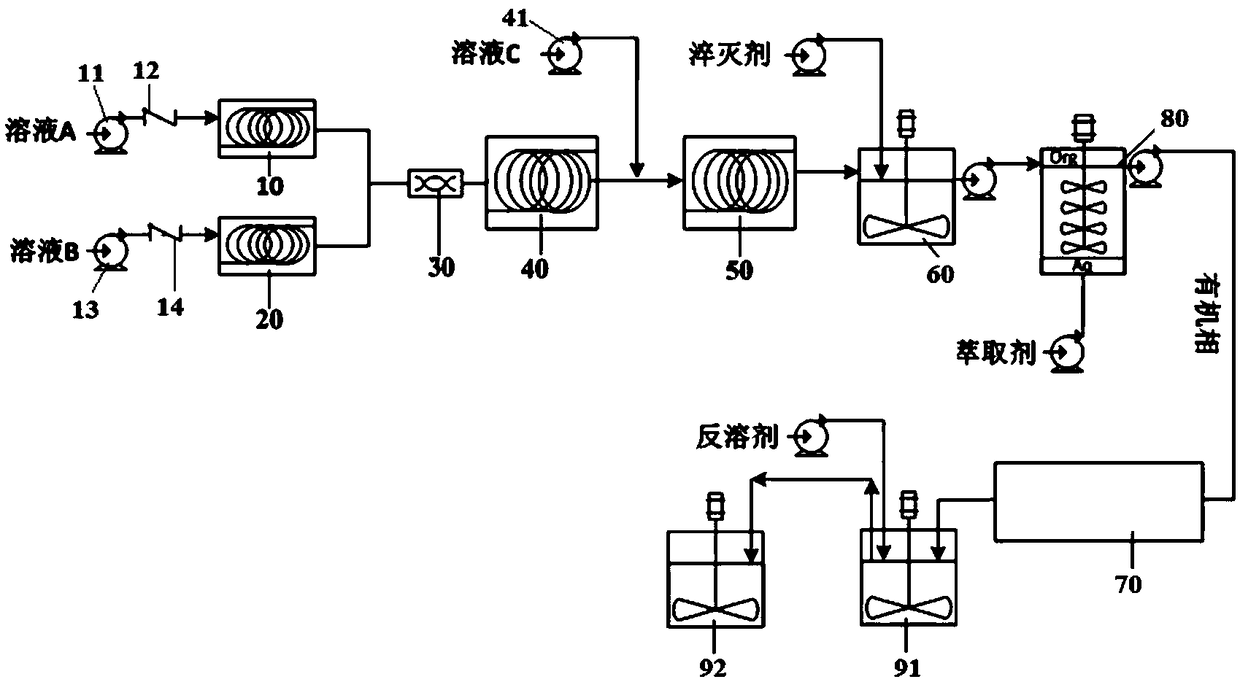
[0092] Dissolve 2-fluorophenylboronic acid (1 equivalent) in the first organic solvent (tetrahydrofuran, 8mL / g), and then add the basic ligand PMDTA (pentamethyldiethylenetriamine, CAS#:3030-47-5, 1.1 Equivalent) mixed uniformly as solution A. n-Butyllithium (22.5 wt% n-hexane solution, 1.6 equivalents) was used as solution B without any dilution. As solution C, isopropyl borate (2.0 equiv) was diluted with THF (2 mL / g).
[0093] according to figure 2 As shown, the continuous reaction equipment is installed and debugged. Solution A, solution B and solution C are sequentially connected to the first material pump 11, the second material pump 13 and the third material pump 41, and the flow rate is automatically controlled by the automatic feeding system. The delivery pipeline of solution A has a first one-way valve 12, and the delivery pipeline of solution B has a second one-way valve 14 (to prevent backflow, solution C decides whether to install a one-way valve as required). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com