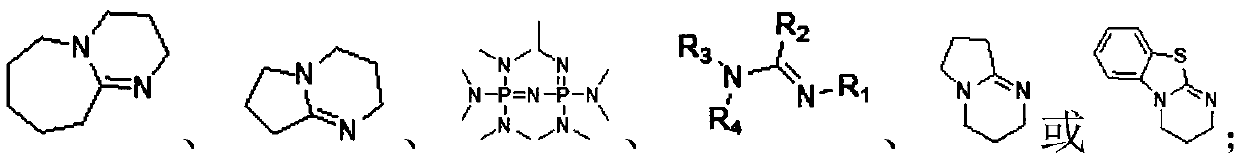
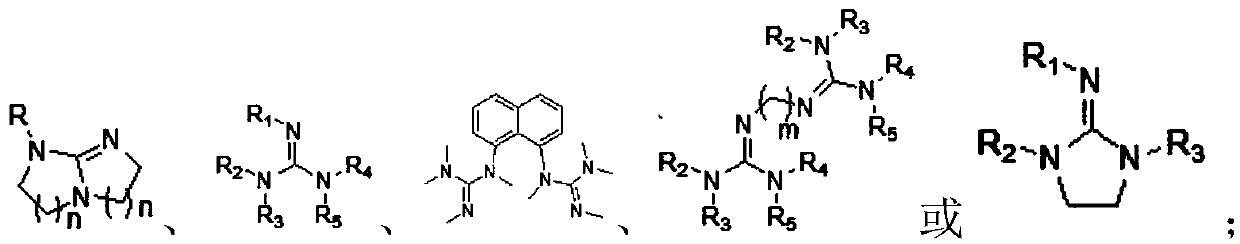
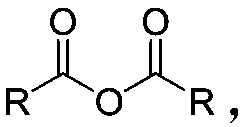
Cellulose levulinate and method for preparing mixed ester with same
A technology of levulinic acid ester and levulinic acid ester, which is applied in the field of compound synthesis, can solve the problems of uncontrollable reaction products, complex product purification energy, difficult to control yield, etc., and achieve efficient, controllable and good transesterification process. The effect of reactivity and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Proceed as follows:
[0064] 1) Mix cellulose, organic base and organic solvent, in a certain CO 2 The pressure is 0.1-10.0MPa, the temperature is 30°C-100°C, and the reaction is 0.1-12 hours to obtain a cellulose solution;
[0065] 2) adding levulinate or angelica lactone to the cellulose solution, and reacting at 30-150° C. for 0.1-48 hours to obtain a cellulose levulinate solution;
[0066] 3) Add C1-C4 lower aliphatic alcohol or ethyl acetate or water to the cellulose levulinate solution, and then filter the reaction mixture containing lower aliphatic alcohol or ethyl acetate or water to obtain a filtered solid; filter The obtained solid mixture is washed, purified and dried with C1-C4 lower aliphatic alcohol or ethyl acetate to obtain cellulose levulinate;
[0067] 4) adding an acylating agent to the cellulose levulinic acid ester solution, and continuing to react at 30-100° C. for 0.5-12 hours to obtain a cellulose levulinic acid mixed ester solution;
[0068] ...
Embodiment 2
[0071] According to the steps of Example 1, 0.5 g of different types of cellulose were weighed, respectively mixed with 1.406 g of 1,8-diazabicyclo[5.4.0]-7-undecene (DBU) and dimethyl methylene Add 7.86g of sulfone (DMSO) into the reactor, cover the reactor, and charge and discharge CO 2 Cycle three times, remove the air, and finally in 0.5Mpa CO 2 Atmosphere, 50° C., stirring for 3 hours to obtain a homogeneous cellulose solution (wt%=5%). Take 10 g of the above solution and add it to a two-neck bottle, and add 2.494 ml (such as formula 2-1) α-angelica lactone dropwise under mechanical stirring at 120°C (the ratio of the molar number of α-angelica lactone to the original -OH on the cellulose 3:1) reaction for 0.5h, the reaction was completed to obtain a dark brown homogeneous solution, using 100mL of isopropanol as an anti-solvent to precipitate a solid product, washed and filtered 3 times, and freeze-dried to obtain the product. The products made from different celluloses...
Embodiment 3
[0077] According to the steps of Example 1, take microcrystalline cellulose 0.5g, take organic base 1,8-diazabicyclo[5.4.0]-7-undecene (DBU), triethylamine (TEA) or 1,5-diazabicyclo[4.3.0]non-5-ene (DBN), take organic solvent dimethylsulfoxide (DMSO), N,N-dimethylformamide (DMF) or N-formaldehyde Pyrrolidone (NMP), one of the microcrystalline cellulose, one of the organic base and one of the organic solvent was mixed and added to the inside of the reaction kettle, the reaction kettle was covered, and the CO 2 Cycle three times, remove the air, and finally in 0.5Mpa CO 2 Atmosphere, 50°C, stirring for 3 hours to obtain a homogeneous cellulose solution. Take 10g of the above solution and put it into a two-neck bottle, add 2.494ml of α-angelica lactone dropwise under mechanical stirring at 120°C (the molar ratio of α-angelica lactone to the original -OH on the cellulose is 3:1) and react for 0.5h , the reaction was completed to obtain a dark brown homogeneous solution, using 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com