Diadema setosum-type PdCuIr nitrogen reduction electrocatalyst and preparation method thereof
An electrocatalyst, sea urchin-like technology, applied in the field of long-needle sea urchin-like PdCuIr nitrogen reduction electrocatalysts and its preparation, can solve the problems of unfavorable and sustainable development, complicated synthesis process, etc., achieve rapid reaction, simple synthesis, and high application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A kind of preparation method of long needle sea urchin shape PdCuIr nitrogen reduction electrocatalyst, described method comprises the steps:
[0040] 1) Prepare a 20mM aqueous solution of sodium chloropalladate, copper chloride and iridium chloride, then mix 2.5mL, 1.0mL and 1.0mL of sodium chloropalladate, copper chloride and iridium chloride respectively;
[0041] 2) 2.0 mL of hydrochloric acid with a concentration of 6M, 2.0 mL of ascorbic acid solution with a concentration of 0.1 M, 200 mg of KBr and 50 mg of F127 were ultrasonically mixed, and ultrasonicated for 10 minutes to form a clear solution;
[0042] 3) Mix the two solutions under stirring, put them in an oil bath and react at 95°C for 30 minutes, after the reaction is completed, wash, centrifuge, and dry to obtain the long-needle sea urchin-like PdCuIr nitrogen-reducing electrocatalyst .
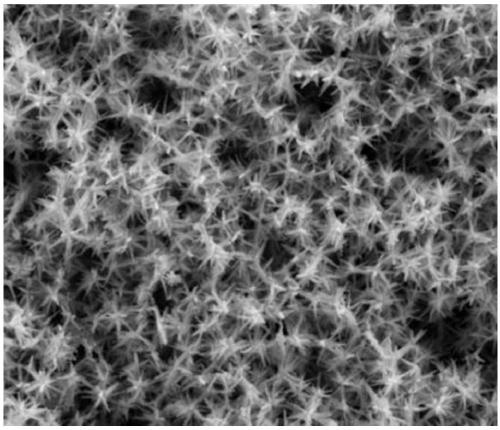
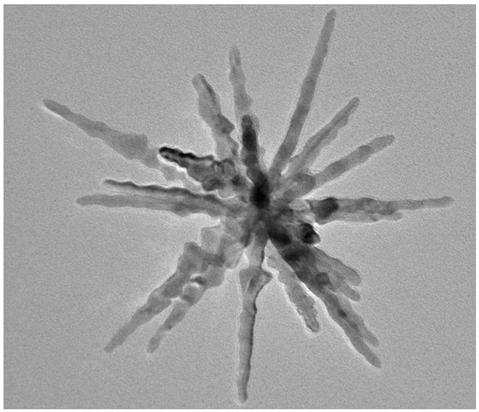
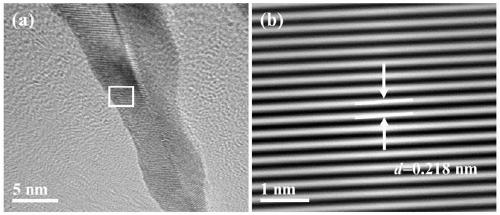
[0043] The SEM picture of the long needle sea urchin shape PdCuIr catalyst obtained sees figure 1 , the TEM image of...
Embodiment 2
[0046] A kind of preparation method of long needle sea urchin shape PdCuIr nitrogen reduction electrocatalyst, described method comprises the steps:
[0047] 1) Prepare a 20mM aqueous solution of sodium chloropalladate, copper chloride and iridium chloride, then mix 1.5mL, 1.5mL and 1.5mL of sodium chloropalladate, copper chloride and iridium chloride solutions;
[0048] 2) 2.0 mL of hydrochloric acid with a concentration of 6M, 2.0 mL of ascorbic acid solution with a concentration of 0.1 M, 200 mg of KBr and 50 mg of F127 were ultrasonically mixed, and ultrasonicated for 10 minutes to form a clear solution;
[0049] 3) Mix the two solutions under stirring, put them in an oil bath and react at 95°C for 60 minutes, after the reaction is completed, wash, centrifuge, and dry to obtain the long-needle sea urchin-like PdCuIr nitrogen-reducing electrocatalyst .
[0050] The SEM picture of the sea urchin shape PdCuIr catalyst that obtains sees Figure 10 , the electric double layer...
Embodiment 3
[0053] A kind of preparation method of long needle sea urchin shape PdCuIr nitrogen reduction electrocatalyst, described method comprises the steps:
[0054] 1) Sodium chloropalladate, cupric chloride and iridium chloride are dissolved in deionized water, and their concentration is 1mM;
[0055] 2) 0.1 mL of hydrochloric acid with a concentration of 1M, 0.5 mL of ascorbic acid solution with a concentration of 0.01 M, 10 mg of KBr and 10 mg of F127 were ultrasonically mixed, and ultrasonicated for 5 minutes to form a clear solution;
[0056] 3) Mix the two solutions under stirring, put them into an oil bath and react at 80° C. for 5 minutes, after the reaction is completed, wash, centrifuge, and dry to obtain the catalyst.
[0057] Due to the low concentration of sodium chloropalladate, copper chloride and iridium chloride solution, few products are obtained, and the amount of ascorbic acid and KBr is very small, it is difficult to completely reduce the Pd and Ru metal sources,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com