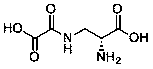
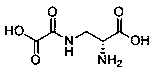
High-efficiency preparation method of D-dencichine
A high-efficiency technology of notoginseng, applied in the field of high-efficiency preparation of D-notoginseng, can solve the problems of unfavorable scale-up production, excessive waste gas, poor solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
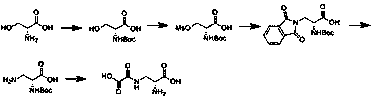
Embodiment 1
[0023] Embodiment 1: the synthesis of Boc-D-serine
[0024] Add 14L of water and 1.37kg of sodium hydroxide to a 50L reaction kettle. Under stirring, add 3kg of D-serine in batches. Add the reaction liquid, react at room temperature for 18 hours, cool down to 0°C, add concentrated hydrochloric acid dropwise, adjust the pH to 2-3.5, add 1.5kg of ethyl acetate for extraction three times, dry and spin to obtain 5kg of colorless oil.
Embodiment 2
[0025] Embodiment 2: the synthesis of Boc-D-serine
[0026] Add 14L of water and 5kg of potassium carbonate to a 50L reaction kettle, and add 3kg of D-serine in batches under stirring. The reaction solution was reacted at room temperature for 18 hours, cooled to 0 degrees, and saturated sodium bisulfate solution was added dropwise to adjust the pH to 2-3.5, and 1.5 kg of ethyl acetate was added to extract three times, dried and then spin-dried to obtain 4 kg of yellow oil.
Embodiment 3
[0027] Embodiment 3: the synthesis of Boc-D-serine mesylate
[0028] In a 10L three-neck flask, add 1kg Boc-D-serine and 5L dichloromethane and stir to dissolve, add 0.6kg triethylamine, cool down to 0 degrees, add 1.12kg methanesulfonyl chloride dropwise, after the dropwise addition, react at room temperature for 8 hours, After the reaction was completed, add 500ml of water to wash, 500ml of saturated sodium bisulfate solution, and 500ml of saturated sodium chloride solution. The organic layer was collected, dried, and spin-dried to obtain 1.13kg of yellow oil.
[0029] Embodiment 3: the synthesis of Boc-D-serine mesylate
[0030] In a 10L three-neck flask, add 1kg Boc-D-serine and 5L tetrahydrofuran and stir to dissolve, add 0.6kg sodium bicarbonate, and add 1.12kg methanesulfonyl chloride dropwise. After the addition is completed, react at room temperature for 8 hours. After the reaction is completed, spin Dry, add 1.5L dichloromethane to the residue, cool to 0-5°C, add 50...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com