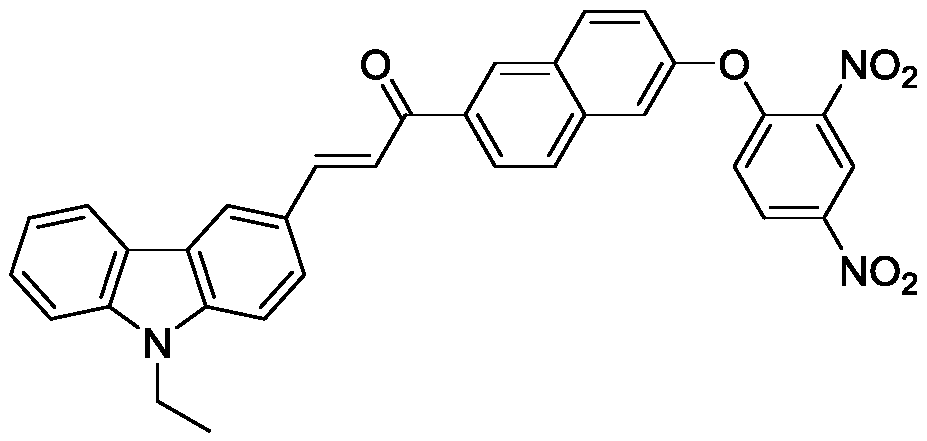
Use of chalcone fluorescent probe in detection of thiophenol compound in water solution
A technology of fluorescent probes and chalcones, applied in the field of analysis, to achieve the effect of simple, fast and accurate experimental methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The design synthesis of embodiment 1 probe molecule probe-KCN1
[0037] Cs was added to a solution of probe-OH (0.5mmol) in dichloromethane (20ml) 2 CO 3 (1 mmol) was stirred at room temperature for 30 min, 2,4-dinitrofluorobenzene (0.625 mmol) was added to the above solution, and stirred overnight at room temperature, a solid precipitated out. The solvent was dried by filtration, and recrystallized from ethyl acetate:petroleum ether (V:V=2:8) to obtain a yellow powdery solid. Yield: 85%.Probe-KCN1: 1 H NMR (500Hz, DMSO-d 6 )δ9.03(s,1H),8.96(d,J=2.7Hz,1H),8.77(s,1H),8.49(dd,J=9.2,2.8Hz,1H),8.37(d,J=8.9 Hz,1H),8.25(d,J=7.9Hz,2H),8.16(d,J=15.4Hz,1H),8.08(d,J=8.7Hz,2H),8.05(d,J=15.5Hz, 1H), 7.89(d, J=2.1Hz, 1H), 7.73(d, J=8.6Hz, 1H), 7.67(d, J=8.2Hz, 1H), 7.60(dd, J=8.9, 2.3Hz, 1H), 7.52(t, J=7.7Hz, 1H), 7.40(d, J=9.2Hz, 1H), 7.29(t, J=7.4Hz, 1H), 4.51(q, J=6.9Hz, 2H) ,1.35(t,J=7.1Hz,3H)ppm; 13 C NMR (125Hz, DMSO-d 6 )δ188.89(s),154.79(s),154.14(s),146.37(s),142.4...
Embodiment 2
[0039] Spectral properties of embodiment 2 probe molecule probe-KCN1
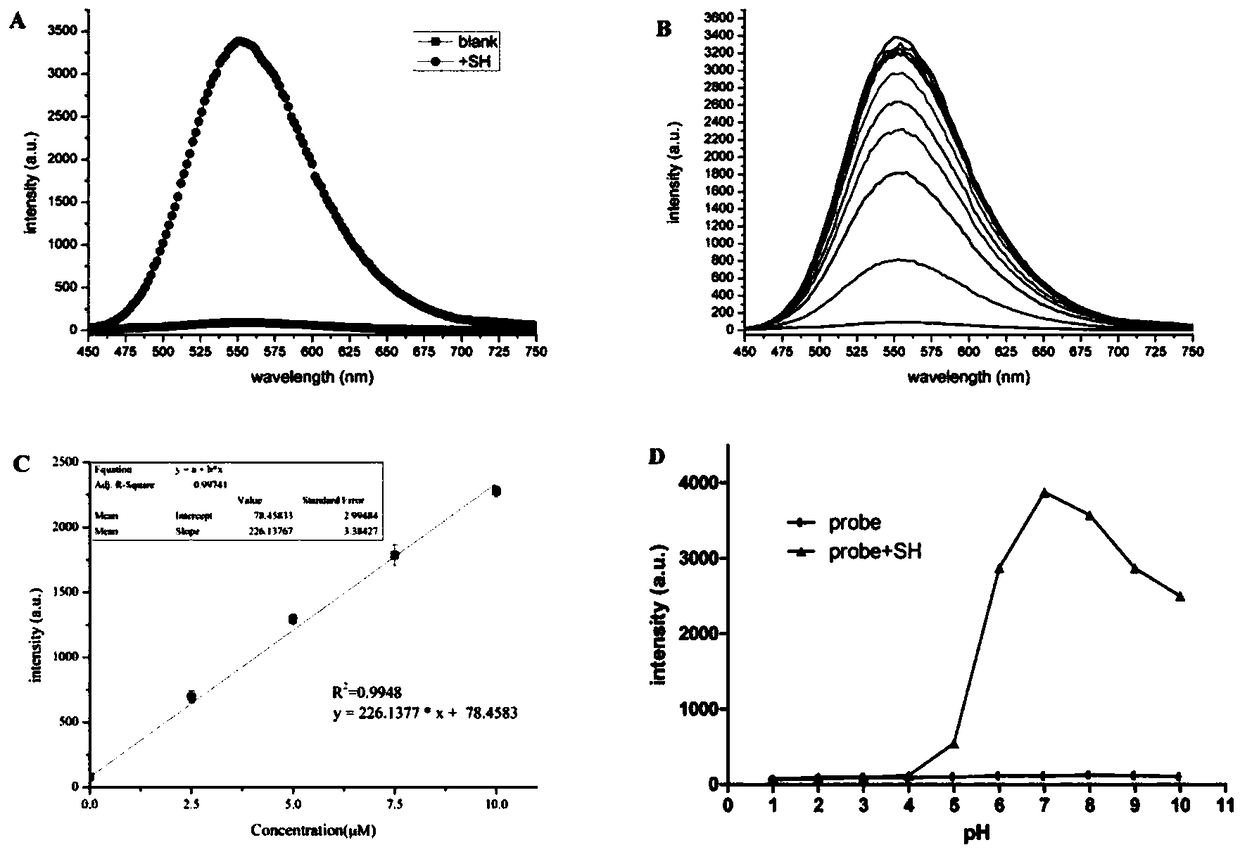
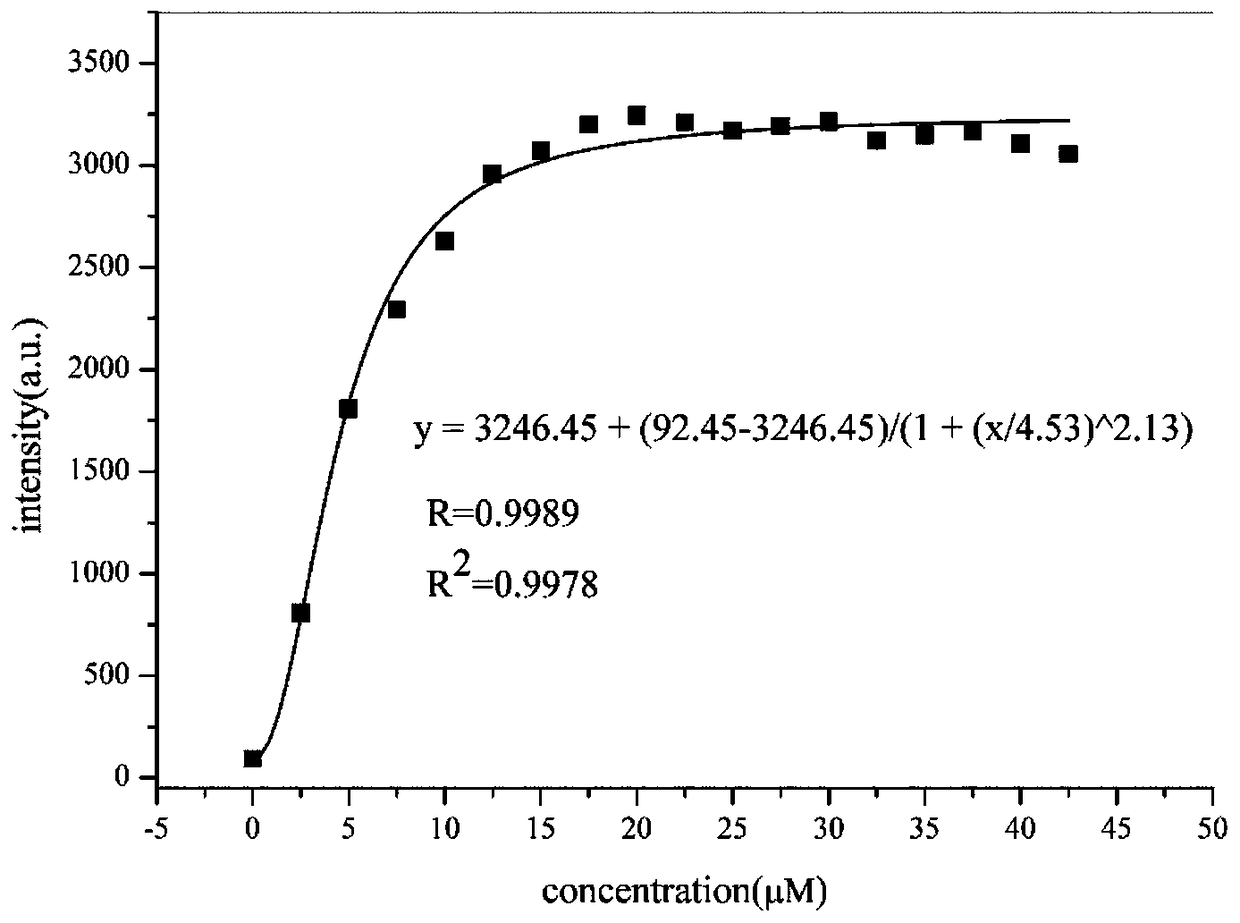
[0040]The experimental method is as follows: the probe molecule probe-KCN1 is dissolved in DMSO to obtain a 5 mM probe mother solution, and stored at 4°C. In the experimental determination, the buffer solution of DMSO / PBS buffer (1:1, v / v, 20mM, pH=7.4) was used to dilute it into a 5μM standard solution. Dissolve 4-methoxythiophenol in DMSO solvent, make 5mM mother solution, store at 4°C, dilute with DMSO / PBSbuffer (1:1, v / v, 20mM, pH=7.4) buffer solution in the experimental measurement Make a standard solution of 0-30μM, set 2.5μM as a concentration gradient. Add 100 μL of probe solution to the 96-well black plate and mix with different concentrations of thiophenol test solution, mix well, incubate at 37°C for 60 minutes, and then measure its spectrum. The results are as follows: figure 2 and image 3 Shown: Adding thiophenol to the probe-KCN1 solution enhanced its fluorescence intensity by 40 times. ...
Embodiment 3
[0046] Example 3 Selectivity experiment of probe molecule probe-KCN1.
[0047] The experimental method is as follows: the probe molecule probe-KCN1 is dissolved in DMSO to obtain a 5 mM probe mother solution, and stored at 4°C. In the experimental determination, the buffer solution of DMSO / PBS buffer (1:1, v / v, 20mM, pH=7.4) was used to dilute it into a 5μM standard solution. 4-Methoxythiophenol, 4-fluorothiophenol and 4-methylthiophenol were dissolved in DMSO solvent to prepare a 2.5mM mother solution, stored at 4°C, and used in DMSO / PBS buffer (1 :1, v / v, 20mM, pH=7.4) buffer solution diluted to 30μM standard solution. Amino acids such as: GSH, Cys, Hcy, Ala, Gly, Thr and Ser; general interfering substances such as: Ph-NH 2 , Ph-OH, NaSH and C 2 h 5 SH; common inorganic salts such as: KI, (AcO) 2 Co,NaHSO 3 ,KBr,SnCl 2 ,FeCl 3 ,CuSO 4 .5H 2 O,Pb(AcO) 4 ,CuI,CaCl 2 ,KF,NaCl,MgSO 4 ,FeSO 4 and NiSO 4 Dissolved in deionized water, configured as a 5mM mother solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com