Method for selectively removing and recovering copper in water by using membrane-assisted crystallization MAC process
A selective, water-recycling technology, applied in chemical instruments and methods, water pollutants, water/sewage treatment, etc., it can solve the problems of MAC process removal and recycling processes that have not been reported, and achieve sustainable social development, reduce Dosage and the effect of avoiding membrane fouling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
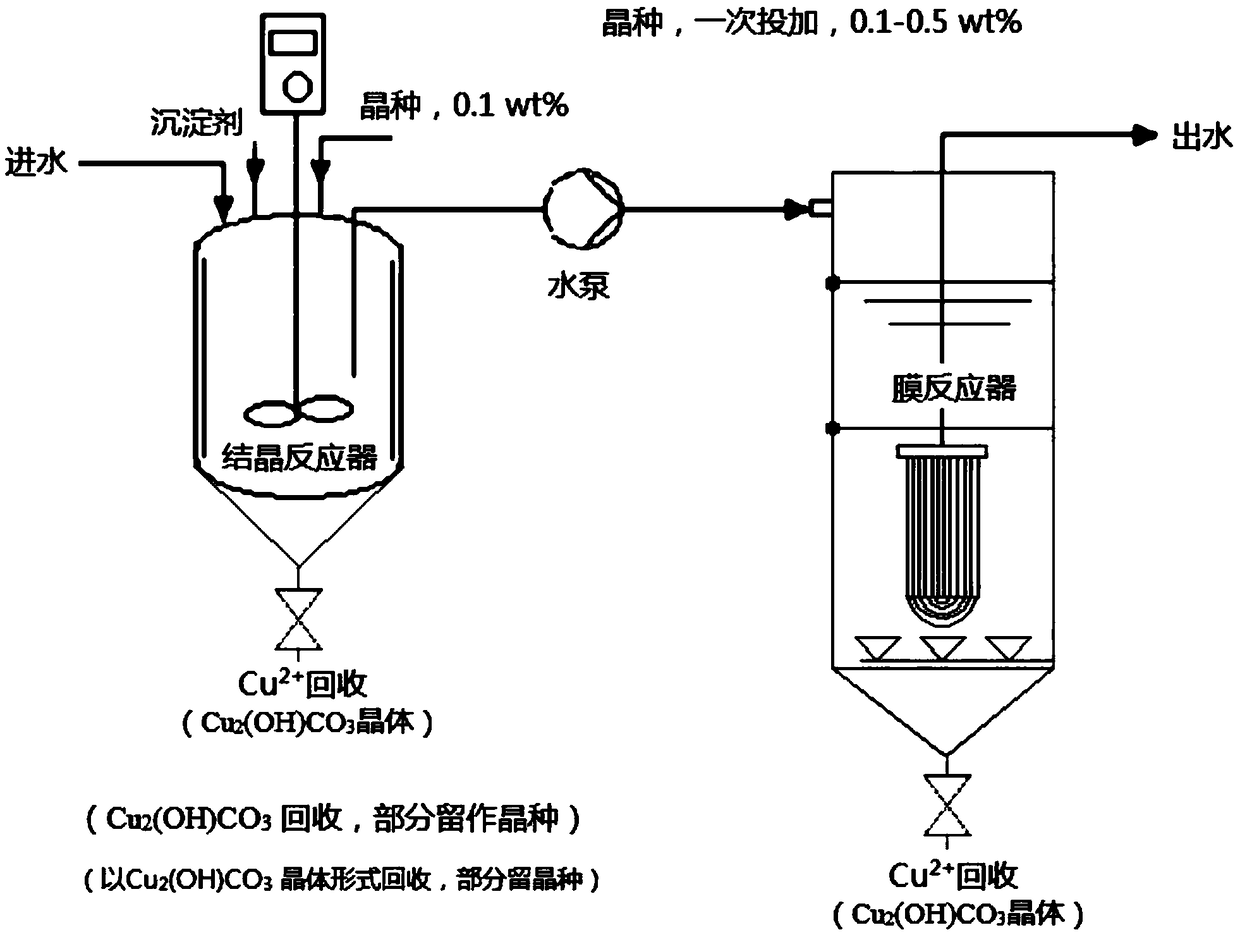
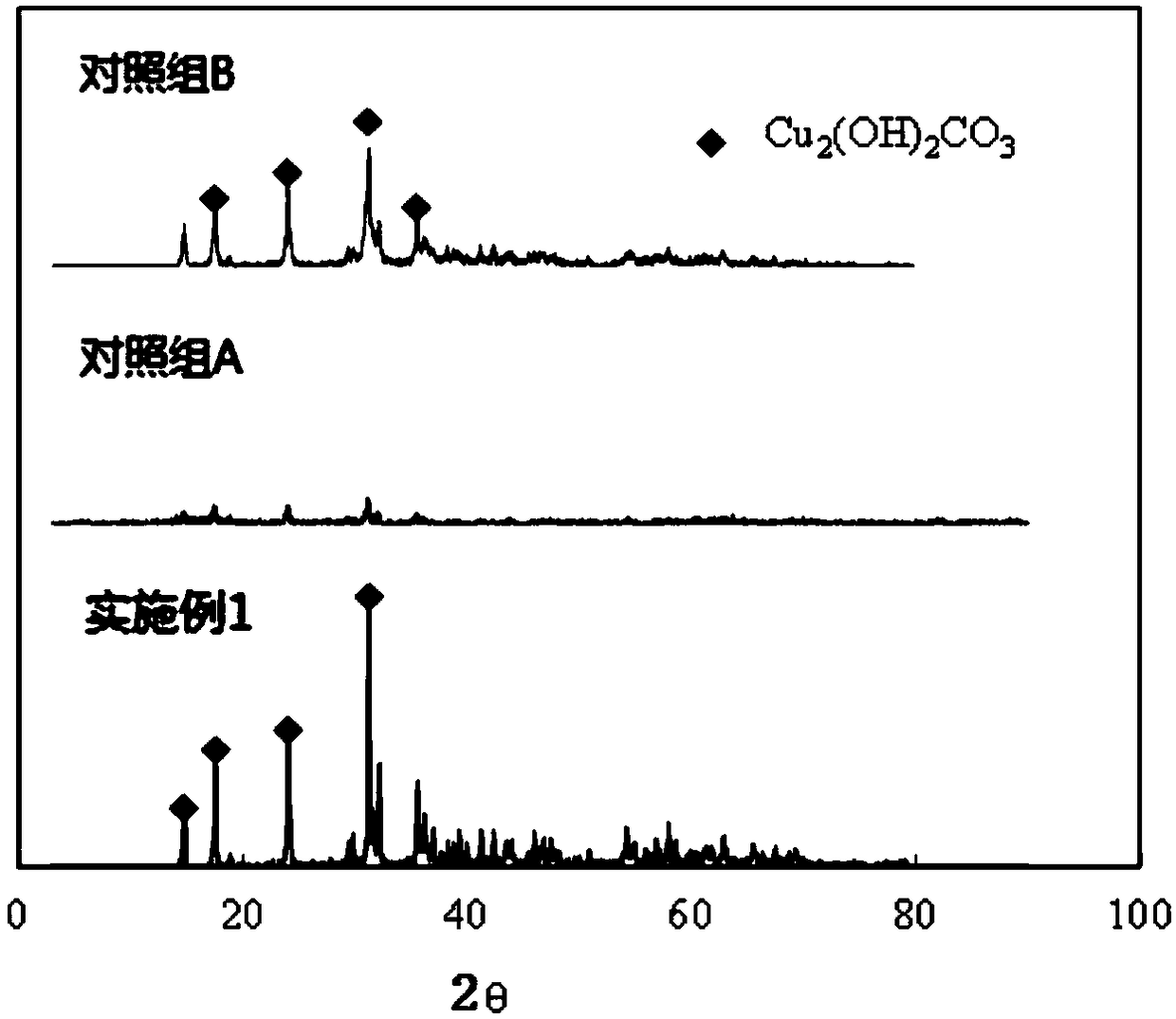
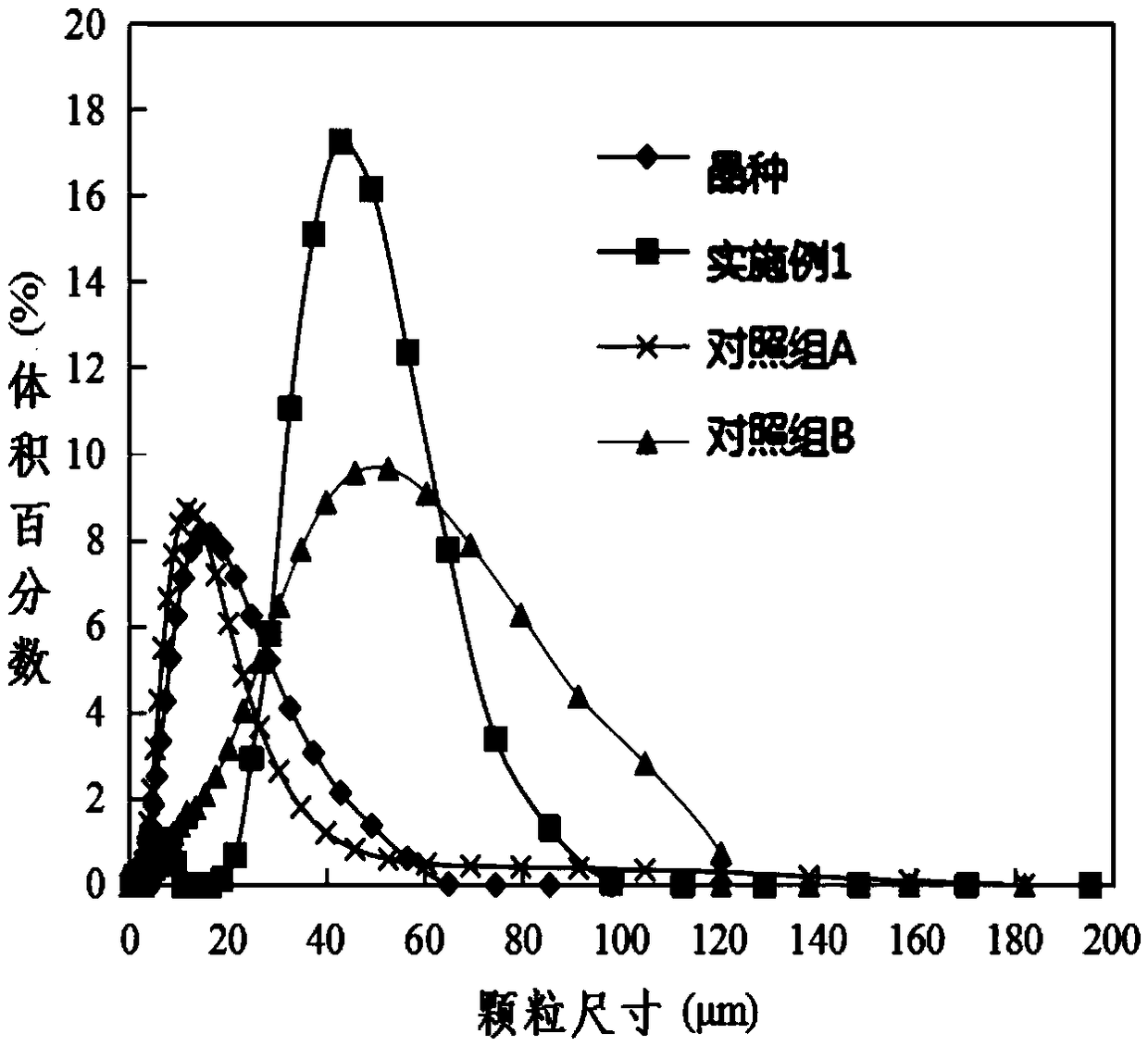
[0085] Embodiment 1: Adopt above-mentioned device to process the copper-containing waste water of 18.9mg / L, this waste water is configured by tap water, wherein, Ca 2+ : 26.3mg / L; Mg 2+ : 9.0 / L; K + : 3.4mg / L; Na + : 12.4mg / L. The treatment water volume of the device is 12.9L / h, the residence time of the crystallization reactor is 13 minutes (5 minutes of stirring, 8 minutes of precipitation), and the hydraulic residence time of the membrane separator is 30 minutes. Before the start of the experiment, 0.1wt% basic copper carbonate seed was added to the crystallization reactor. When the calculated sodium carbonate dosage was controlled at 104mg / L, the SI value was 2.76, and the remaining carbonate ion concentration was 41.1mg / L At this time, the copper concentration of the MAC process effluent is stable below 0.8mg / L, the turbidity is stable at 0.1NTU, and the pH of the effluent is 7.62±0.1. After treating 1800L of water, the recovered product is 0.24ml, that is, the concen...
Embodiment 2
[0088] Embodiment 2: Adopt above-mentioned device to process the copper-containing waste water of 29.5mg / L, this waste water is configured by tap water, wherein, Ca 2+ : 33.0mg / L; Mg 2+ : 8.9mg / L; K + : 3.4mg / L; Na + : 19..4mg / L. The treatment water volume of the device is 15L / h, the residence time of the crystallization reactor is 15 minutes (8 minutes of stirring, 15 minutes of precipitation), and the hydraulic residence time of the membrane separator is 40 minutes. Before the start of the experiment, 0.2wt% basic copper carbonate seeds were added to the crystallization reactor. When the calculated sodium carbonate dosage was controlled at 115mg / L, the SI value was 3.17, and the remaining carbonate ion concentration was 37.8mg / L At this time, the copper concentration of the MAC process effluent is stable below 1.295mg / L, the turbidity is stable at 0.1NTU, the pH of the effluent is 7.42±0.1, and the recovered product is dense Cu 2 (OH) 2 CO 3 crystal particles.
Embodiment 3
[0089] Embodiment 3: Adopt above-mentioned device to process the copper-containing waste water of 29.5mg / L, this waste water is configured by tap water, wherein, Ca 2+ : 33.0mg / L; Mg 2+ : 8.9mg / L; K + : 3.4mg / L; Na + : 19..4mg / L. The treatment water volume of the device is 15L / h, the residence time of the crystallization reactor is 15 minutes (8 minutes of stirring, 15 minutes of precipitation), and the hydraulic residence time of the membrane separator is 40 minutes. Before the experiment was started, 0.2wt% basic copper carbonate seeds were added to the crystallization reactor. When the calculated sodium carbonate dosage was controlled at 95mg / L, the SI value was 3.12, and the remaining carbonate ion concentration was 31.1mg / L At this time, the copper concentration of the MAC process effluent is stable below 1.895mg / L, the turbidity is stable at 0.1NTU, the pH of the effluent is 7.32±0.1, and the recovered product is dense Cu 2 (OH) 2 CO 3 crystal particles.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com