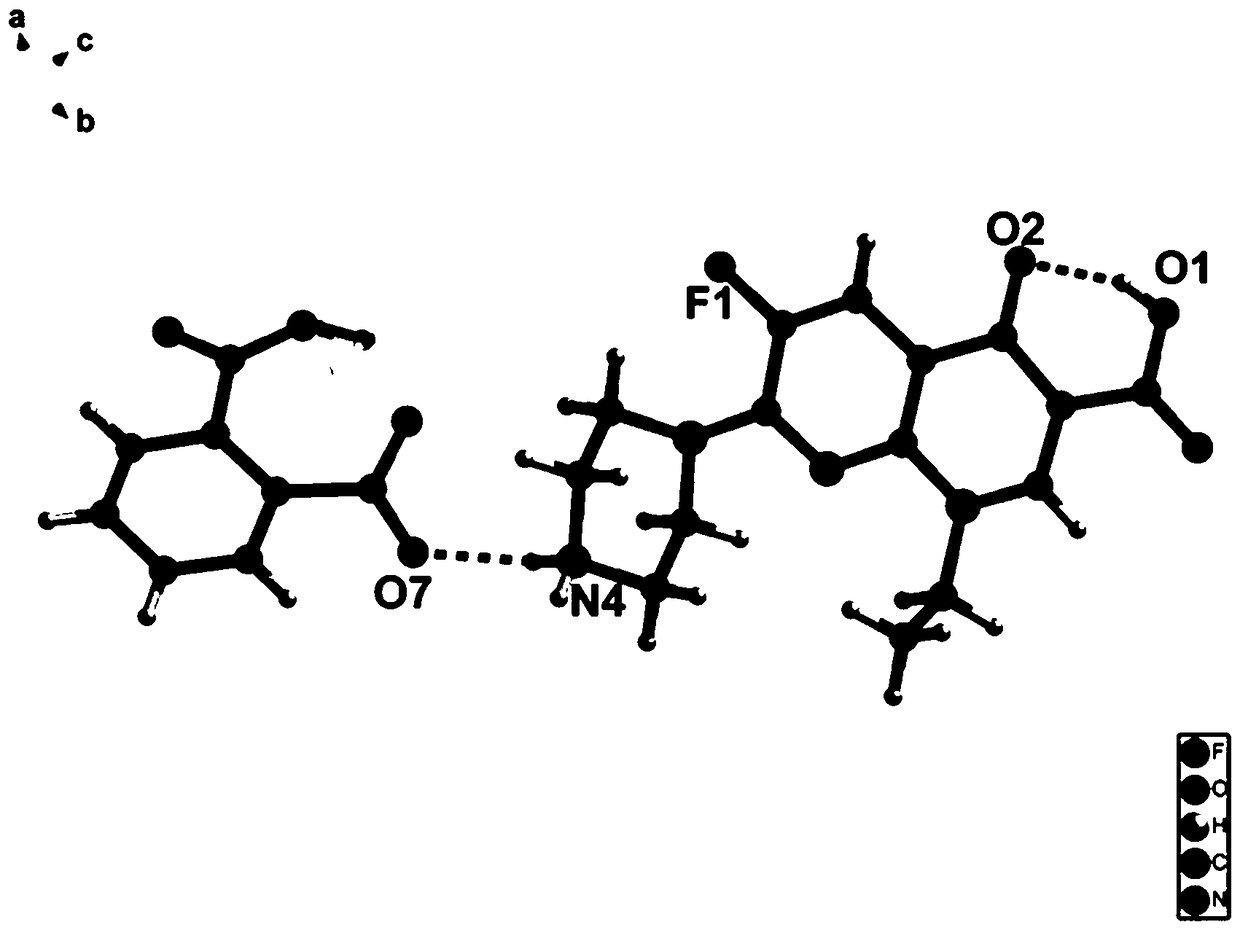
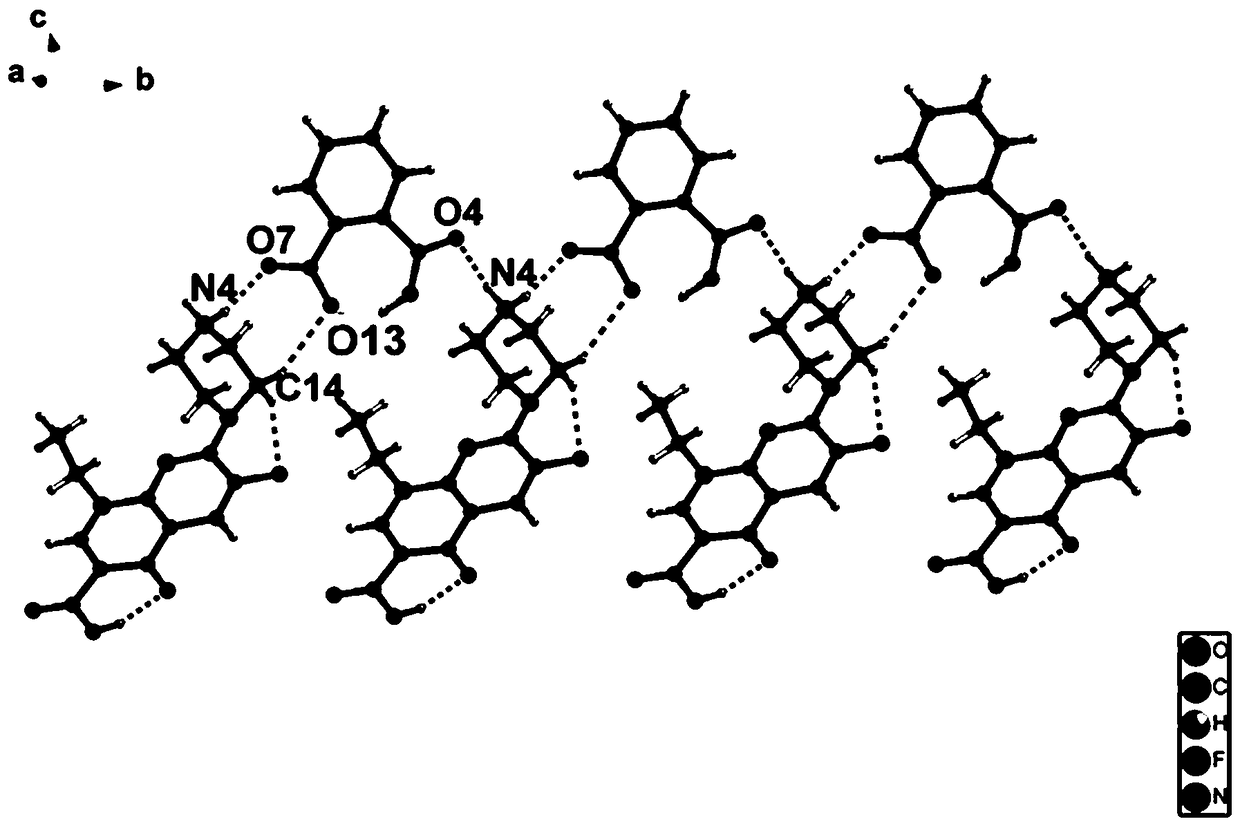
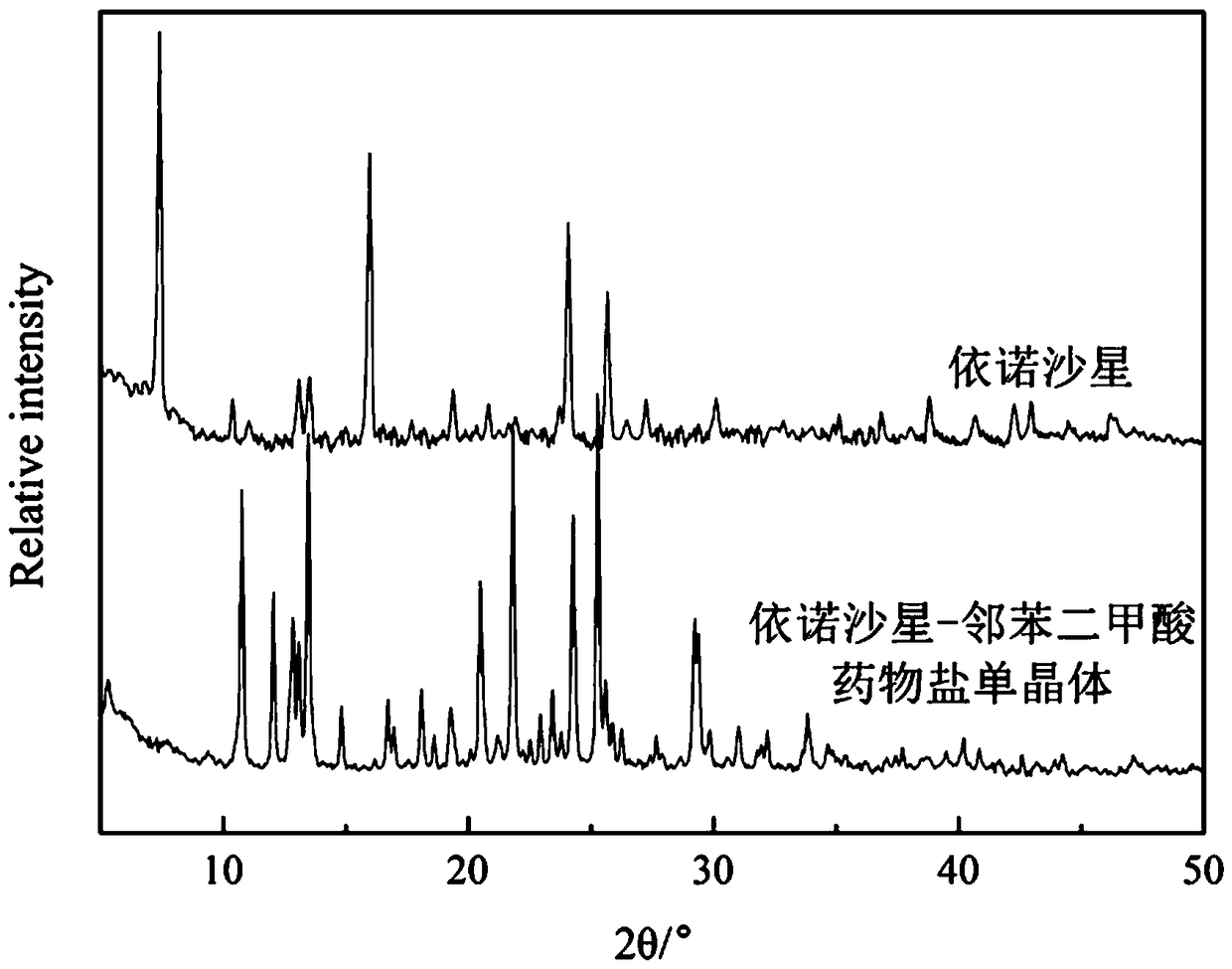
Enoxacin-phthalic acid drug salt monocrystal and preparation method thereof
A technology of phthalic acid and enoxacin, which is applied in the field of enoxacin pharmaceutical salt single crystal, can solve the problems of affecting the exertion of drug efficacy and clinical application, unsatisfactory uniformity and stability, low bioavailability and the like, Achieve significant clinical application value, avoid deterioration or mildew, and improve solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A preparation method of the enoxacin-phthalic acid drug salt single crystal, enoxacin 0.05mmol and phthalic acid 0.15mmol are placed together in a glass container at a molar ratio of 1:3, and then jointly Dissolve in a mixed solution of water (5mL) and methanol (5mL), place the glass container containing the mixed solution on a heating stirrer, and stir in a constant temperature water bath at 45°C for 3 hours. After fully reacting, dry Slowly volatilize under the environment, and after 1-30 days, a colorless block crystal is formed, which is the single crystal of enoxacin-phthalic acid drug salt.
Embodiment 2
[0028] A preparation method of the enoxacin-phthalic acid pharmaceutical salt single crystal, enoxacin 0.1mmol and phthalic acid 0.3mmol are placed together in a glass container at a molar ratio of 1:3, and then jointly Dissolve in a mixed solution of ethanol (7mL) and water (3mL), place the glass container containing the mixed solution on a heating stirrer, and stir in a constant temperature water bath at 40°C for 3.5 hours. After fully reacting, dry Slowly volatilize under the environment, and after 1-30 days, a colorless block crystal is formed, which is the single crystal of enoxacin-phthalic acid drug salt.
Embodiment 3
[0030] A preparation method of the enoxacin-phthalic acid drug salt single crystal, enoxacin 0.05mmol and phthalic acid 0.15mmol are placed together in a glass container at a molar ratio of 1:3, and then jointly Dissolve in a mixed solution of ethanol (9mL) and water (1mL), place the glass container containing the mixed solution on a heating stirrer, and stir in a constant temperature water bath at 45°C for 3 hours. After fully reacting, dry Slowly volatilize under the environment, and after 1-30 days, a colorless block crystal is formed, which is the single crystal of enoxacin-phthalic acid drug salt.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com