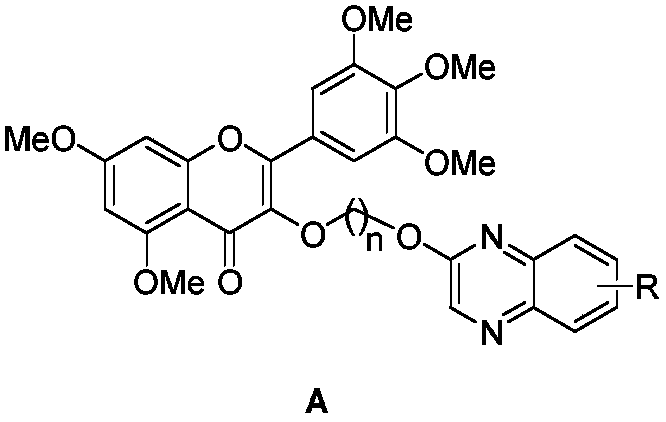
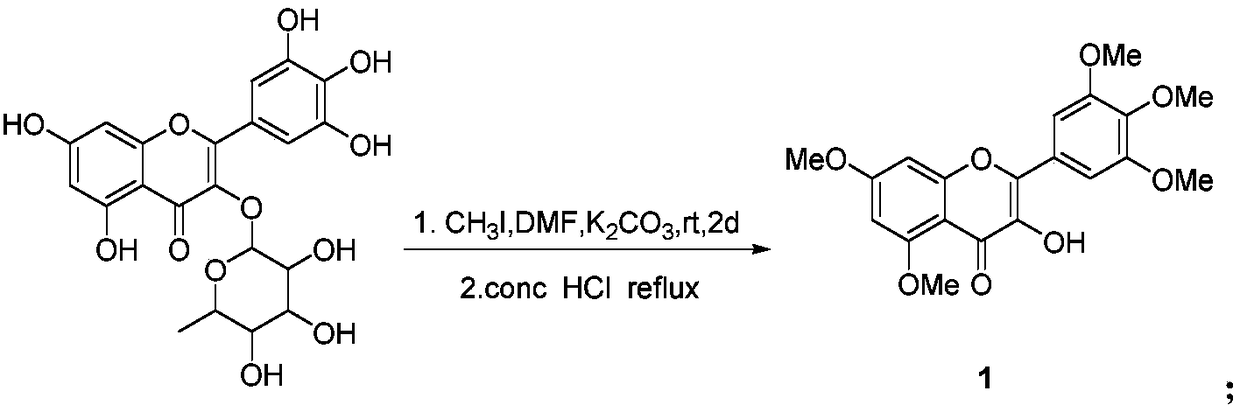
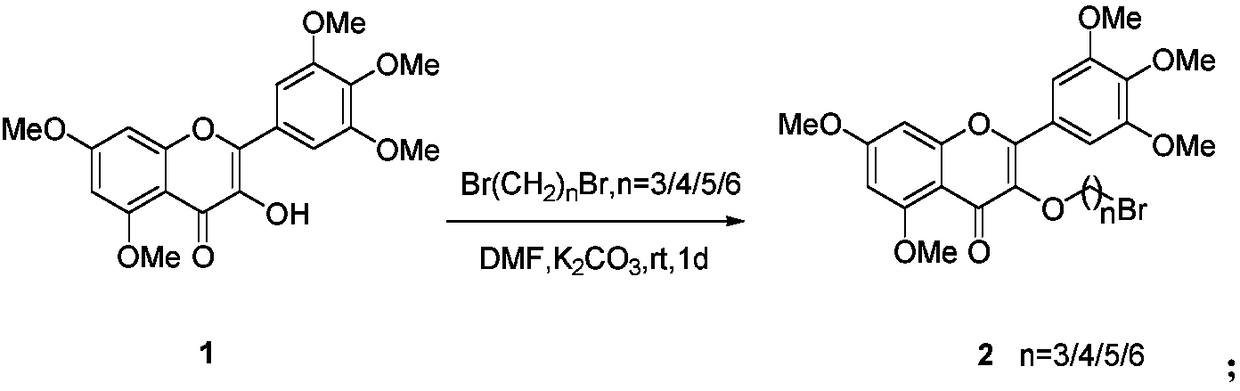
Myricetin derivative containing quinoxaline and preparation method and application thereof
A kind of derivative, the technology of quinoxaline, applied in the field of myricetin derivatives, to achieve the effect of good anti-plant virus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 5,7-Dimethoxy-3-(3-(quinoxalin-2-yloxy)propoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromene -4-ketone (target compound A 1 ) preparation method, comprising the following steps:
[0026] (1) Preparation of 3-hydroxy-3',4',5',5,7-pentamethoxymyricetin
[0027] In a 250mL round bottom flask, add 4.64g myricetin (10mmol), 22.09g K 2 CO 3 (16mmol) and 120mL DMF, stirred at room temperature for 0.5~1h, slowly added 7.50mL iodomethane (120mmol) dropwise, stirred at room temperature for 48h, TLC followed the reaction (methanol:ethyl acetate=1:4, V / V). After stopping the reaction, filter the precipitate, wash the filter residue with dichloromethane, combine the filtrates, dilute with 100mL water, or extract three times with dichloromethane, combine the organic layers, concentrate under reduced pressure, and then dissolve the concentrate in 30mL absolute ethanol , heated to reflux, after the solution was clarified, 16mL of concentrated hydrochloric acid was added under reflux, and...
Embodiment 2
[0033] 3-(3-(3-Hydroxyquinoxalin-2-yl)oxy)propoxy-5,7-dimethoxy-2-(3,4,5-trimethoxyphenyl)-4H -Chromene-4-one (target compound A 2 ) preparation method, comprising the following steps:
[0034] (1) Preparation of 3-hydroxy-3',4',5',5,7-pentamethoxymyricetin
[0035] As in the first (1) step of Example 1.
[0036] (2) Preparation of 3(3-bromopropoxy)-5,7-dimethoxy-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one
[0037] As in embodiment 1 (2) step.
[0038] (3) 3-(3-(3-hydroxyquinoxalin-2-yl)oxy)propoxy-5,7-dimethoxy-2-(3,4,5-trimethoxyphenyl )-4H-chromen-4-one preparation
[0039] As in step (3) of Example 1, the difference is that 2,3-dihydroxyquinoxaline is used as a raw material. Yield: 52%.
Embodiment 3
[0041] 5,7-Dimethoxy-3-(3-((3-methylquinoxalin-2-yl)oxy)propoxy)-2-(3,4,5-trimethoxyphenyl )-4H-chromen-4-one (target compound A 3 ) preparation method, comprising the following steps:
[0042] (1) Preparation of 3-hydroxy-3',4',5',5,7-pentamethoxymyricetin
[0043] As in the first (1) step of Example 1.
[0044] (2) Preparation of 3(3-bromopropoxy)-5,7-dimethoxy-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one
[0045] As in embodiment 1 (2) step.
[0046] (3) 5,7-dimethoxy-3-(3-((3-methylquinoxalin-2-yl)oxy)propoxy)-2-(3,4,5-trimethoxy Preparation of phenyl)-4H-chromen-4-one
[0047] As in step (3) of Example 1, the difference is that 2-hydroxyl-3-methylquinoxaline is used as a raw material. Yield: 63%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com