A kind of preparation method of bicyclic cationic complex metal iridium-pyridazine crystal material
A cationic, crystalline material technology, applied in luminescent materials, indium organic compounds, organic chemical methods, etc., can solve problems such as short phosphorescence lifetime, achieve laser protection, improve nonlinear optical performance, and good nonlinear optical absorption effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
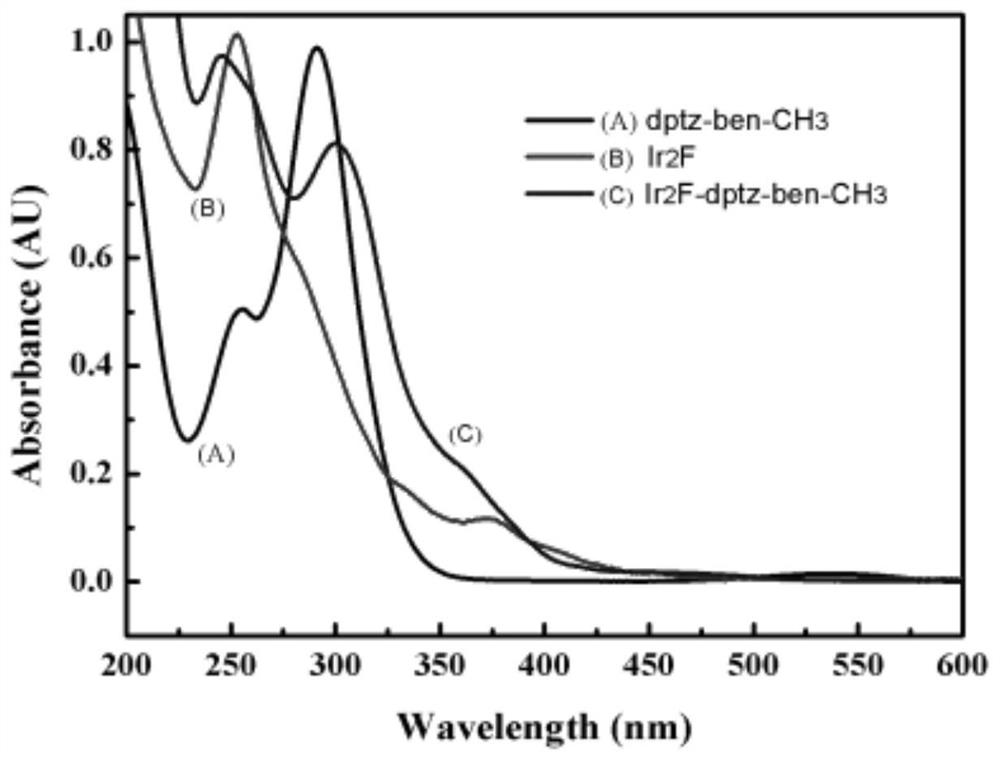
Image
Examples
Embodiment 1
[0036] Step 1: Iridium dichloro bridge compound [Ir(dfppy) 2 (μ 2 -Cl)] 2 Synthesis
[0037] Add hydrated iridium trichloride (350mg, 1mmol) and 2-(2,4-difluoro) phenylpyridine ligand (0.5ml, 2.5mmol), ethylene glycol ether 45mL and water 15mL in the gram flask, the flask Heat and reflux at 135° C. for 24 hours under the condition of sealing, vacuuming and nitrogen protection. During the reaction, the temperature is strictly controlled and isolated from the air. Cool to room temperature. Suction filtration was carried out with a small cloth funnel, and the obtained precipitate was washed with ethanol and petroleum ether to remove reactants and impurities. CH 2 Cl 2 Extraction and further purification yielded the target product as a light yellow powder, which was dried in vacuum and weighed (385 mg, 30%).
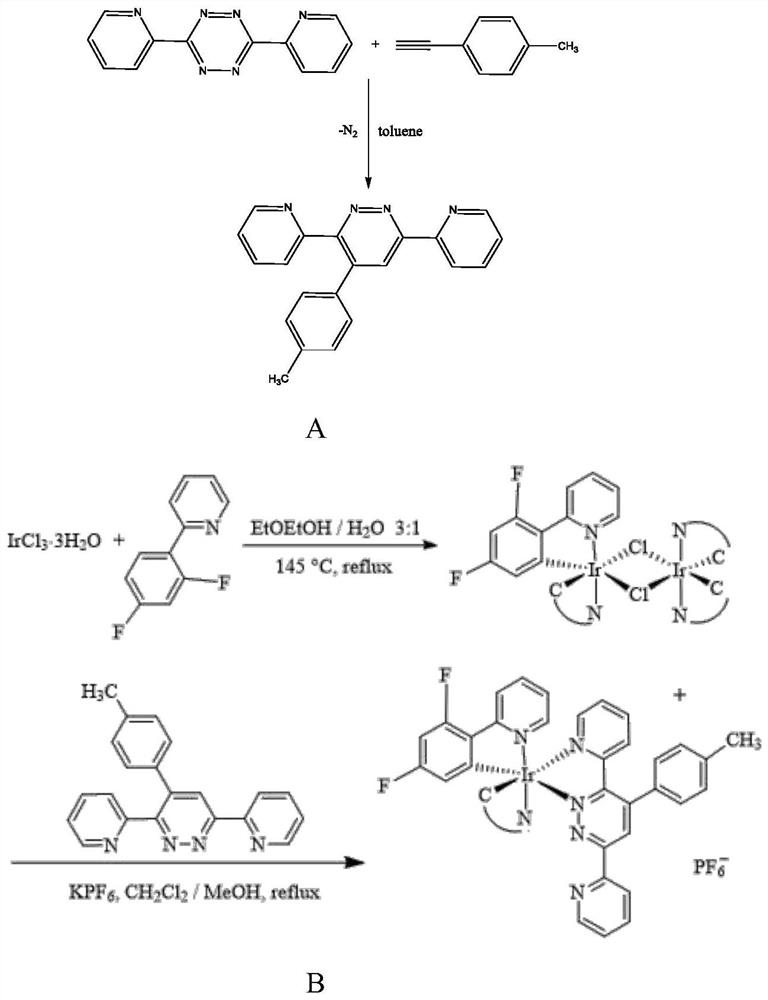
[0038] Step 2: Synthesis of auxiliary ligands, the synthetic route is as follows figure 1 (A)
[0039] Take 4-ethynylaniline (0.40g, 3.4mmol) and 3,6-bis(2-pyridyl)-1...
Embodiment 2
[0045] Step 1 and step 2 are the same as embodiment 1;
[0046] Step 3: In a 100ml reaction flask, put 60mg of 3,6-bis-2-pyridyl-4-methylphenylpyridazine and 120mg of fluorine metal iridium dimer and add 40ml of dichlorohexane and 15ml of methanol , and then 60 mg of potassium hexafluorophosphate was added thereto. Nitrogen was blown into the bottle for 15 minutes for protection, then the temperature was raised in an oil bath at 85°C, and the reaction was stirred and refluxed for 24 hours. The reaction solution was added with silica gel and spin-dried, and finally separated by column chromatography (dichloromethane-ethanol), and the ribbon was collected by spotting and spin-dried to obtain 68 mg of metal iridium complex solid.
[0047] Step 4: Dissolve the product of step 3 in dichloromethane according to the ratio of metal iridium complex solid: dichloromethane: buffer layer: n-hexane = 1mmol: 1mL: 1mL: 2mL, and then slowly dropwise add buffer layer, and then slowly drop n-...
Embodiment 3
[0049] Step 1 and step 2 are the same as embodiment 1;
[0050] Step 3: In a 100ml reaction flask, put 60mg of 3,6-bis-2-pyridyl-4-methylphenylpyridazine and 120mg of fluorine metal iridium dimer and add 40ml of dichlorohexane and 15ml of methanol , and then 70 mg of potassium hexafluorophosphate was added thereto. Nitrogen was blown into the bottle for 15 minutes for protection, then the temperature was raised in an oil bath at 85°C, and the reaction was stirred and refluxed for 24 hours. The reaction solution was added with silica gel and spin-dried, and finally separated by column chromatography (dichloromethane-ethanol), and the ribbon was collected by spotting and spin-dried to obtain 62 mg of metal iridium complex solid.
[0051] Step 4: Dissolve the product of step 3 in dichloromethane according to the ratio of metal iridium complex solid: dichloromethane: buffer layer: n-hexane = 1mmol: 1mL: 1mL: 2mL, and then slowly dropwise add buffer layer, and then slowly drop n-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com