Complete humanized monoclonal antibody for RSV attachment G protein surface antigen
A monoclonal antibody, fully human technology, applied in the direction of antibodies, immunoglobulins, antibody medical components, etc., to achieve the effect of reducing viral load, high affinity, and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation of fully human anti-RSV antibodies RSVG71 and RSVG78
[0028] 1. Artificially synthesized heavy and light chains of RSVG71 and RSVG78
[0029] According to the nucleic acid sequence of the heavy chain of the monoclonal antibody RSVG71 shown in SEQ ID NO:1, the light chain (KAPPA) of the monoclonal antibody RSVG71 shown in SEQ ID NO:2; as shown in SEQ ID NO:3 The nucleic acid sequence of the heavy chain of the monoclonal antibody RSVG78 shown in SEQ ID NO: 4 and the light chain (KAPPA) of the monoclonal antibody RSVG78 (KAPPA) was sent to GENEWIZ Company for artificial synthesis.
[0030]The artificially synthesized RSVG71 and RSVG78 heavy chains and light chains were used as templates, and Taq enzyme, dNTPs, and primers were added, respectively, for PCR to obtain PCR products.
[0031] 2. Construction of expression vectors for recombinant antibodies
[0032] The PCR product was recovered using a rapid DNA product purification kit (purchased from ...
Embodiment 2
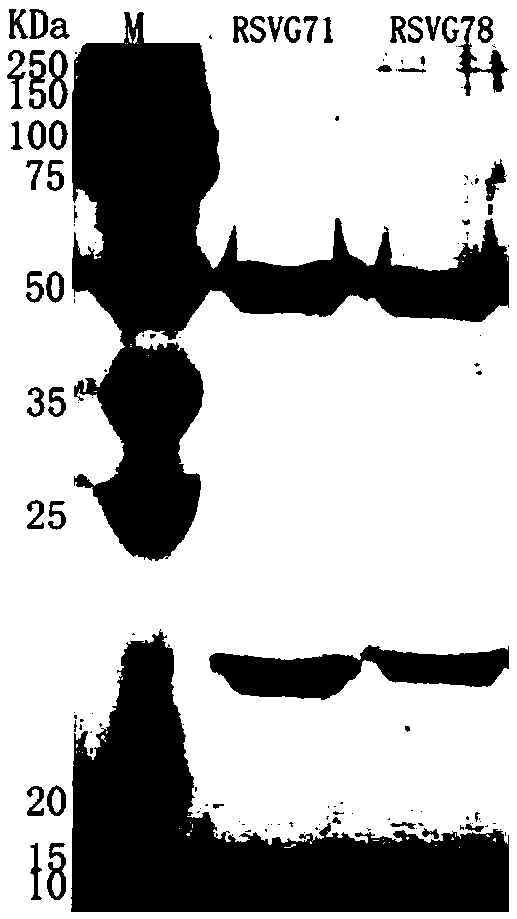
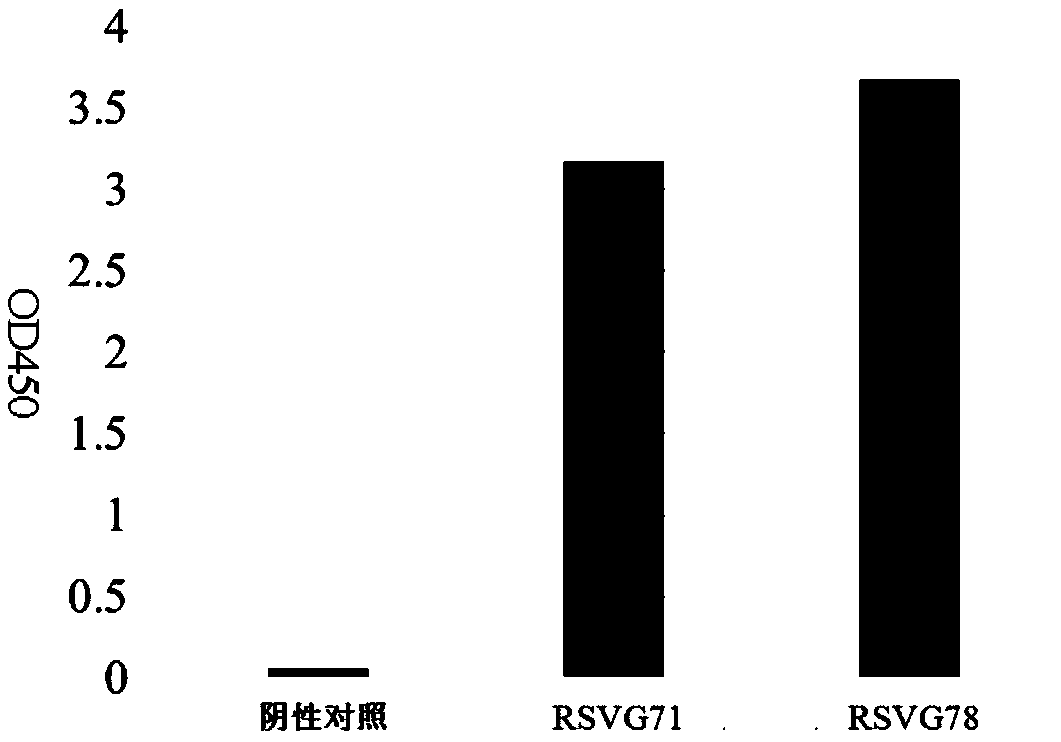
[0040] Expression and purification of embodiment 2 monoclonal antibodies RSVG71 and RSVG78
[0041] Using the plasmid containing the heavy chain (RH71) of RSV G71 obtained in Example 1 and the plasmid containing the light chain (RK71) of RSV G71, and the plasmid containing the heavy chain (RH78) of RSV G78 and the light chain (RK78) containing RSV G78 The plasmids were transfected into 293T cells. Dilute the plasmid and PEI with Opti-MeM (1X) buffer, then slowly add the PEI-Opti-MeM mixture into the plasmid-Opti-MeM mixture tube, let it stand at room temperature for 20 minutes, then add the PEI and plasmid mixture to the in the cell suspension. The cell concentration at the time of transfection was 0.25~0.5×10 6 cells / ml, transfection of cells per well uses 2.5 μg plasmid containing RSV G71 heavy chain + 2.5 μg plasmid containing RSV G71 light chain + 10 μg PEI, and 2.5 μg plasmid containing RSV G78 heavy chain + 2.5 μg plasmid containing RSV G78 light chain Plasmid + 10 μg...
Embodiment 3
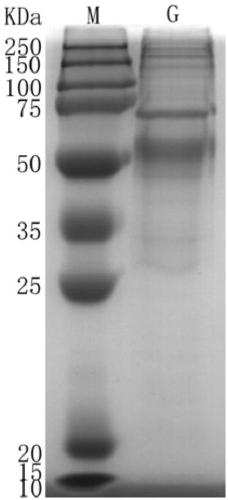
[0044] Example 3 Expression and purification of RSV G antigen
[0045] (1) Construction of G protein expression vector
[0046] First, the G protein target sequence is artificially synthesized by genetic engineering, and 6 His are added to the N-terminus of the sequence, which can be combined with nickel in the nickel column, so that it can be purified by affinity chromatography, and NheI / NotI are added at both ends Two enzyme cutting sites. Both the synthesized G protein DNA fragment and the expression vector pcDNA3.1-Zeo(+) (Invitrogen Company) were digested with NheI / NotI, and the G protein target fragment and the expression vector fragment were recovered, ligated, transformed, and PCR and Positive clones were identified by restriction enzyme digestion, and the correctness of the expression vector was finally verified by sequencing. A large number of plasmids were extracted by alkaline lysis for transient transfection.
[0047] (2) Transient transfection of 293F cells
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com