Cladribine injection and preparation method thereof
A technology of injection and phosphate solution, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the unstable state of raw materials, the growth of impurities in injections, and the increase in operating costs and other issues to achieve the effect of increasing market share, stabilizing drug efficacy, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation process of cladribine injection follows the steps below:
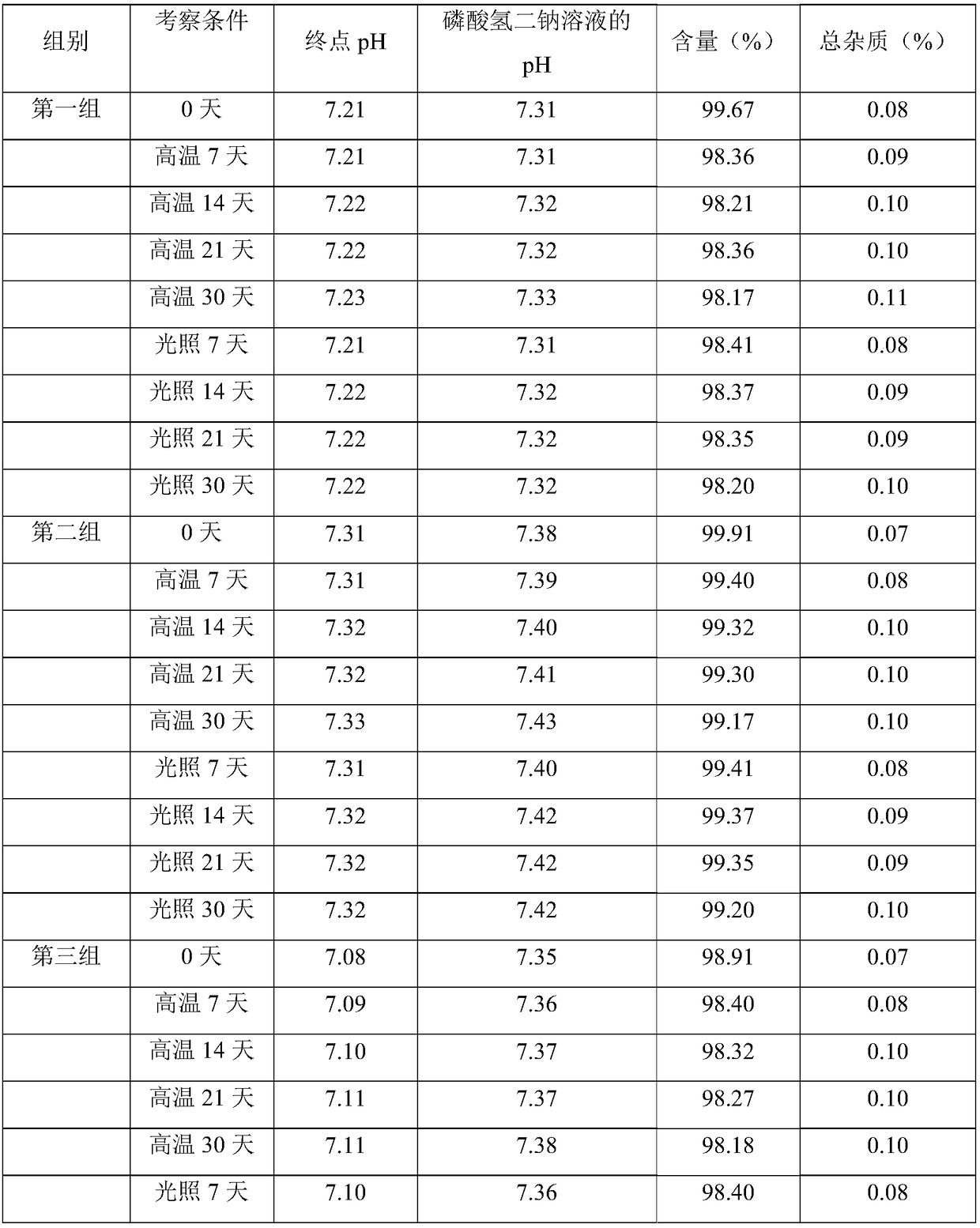
[0043] 1. Prepare a 0.01mol / L disodium hydrogen phosphate solution: anhydrous disodium hydrogen phosphate is dissolved in water for injection to a constant volume, and the pH is adjusted to 7 with phosphoric acid;
[0044] 2. Sodium chloride and cladribine are mixed at a mass ratio of 9:1, dissolved in 0.01mol / L disodium hydrogen phosphate solution, and the pH value of the solution is detected, and the dissolution temperature is controlled at 20°C;
[0045] 3. Check that the pH is greater than 7, adjust the pH to 7 with phosphoric acid solution, then add 0.01mol / L disodium hydrogen phosphate solution to continue to adjust the pH to 7;
[0046] 4. Check that the pH is less than 7, add 0.01mol / L disodium hydrogen phosphate solution and continue to adjust the pH to 7;
[0047] 5. Constant volume, packaging, testing, sterilization, sealing, and storage in a cool place (not exceeding 20°C) with shadi...
Embodiment 2
[0055] The preparation process of cladribine injection follows the steps below:
[0056] 1. Prepare 0.015mol / L disodium hydrogen phosphate solution: anhydrous disodium hydrogen phosphate is dissolved in water for injection to a constant volume, and the pH is adjusted to 8 with phosphoric acid;
[0057] 2. Dissolve calcium chloride with 0.015mol / L disodium hydrogen phosphate solution and stir, then add cladribine and continue stirring. Calcium chloride and cladribine are mixed in a mass ratio of 10:1, and the pH of the solution is detected value, the temperature of dissolution is controlled at 25°C;
[0058] 3. Check that the pH is greater than 7, adjust the pH to 7 with 1.5wt% phosphoric acid solution, and then add 0.015mol / L disodium hydrogen phosphate solution to continue to adjust the pH to 8;
[0059] 4. Check that the pH is less than 7, add 0.015mol / L disodium hydrogen phosphate solution and continue to adjust the pH to 8;
[0060] 5. Constant volume, packaging, testing...
Embodiment 3
[0062] The preparation process of cladribine injection follows the steps below:
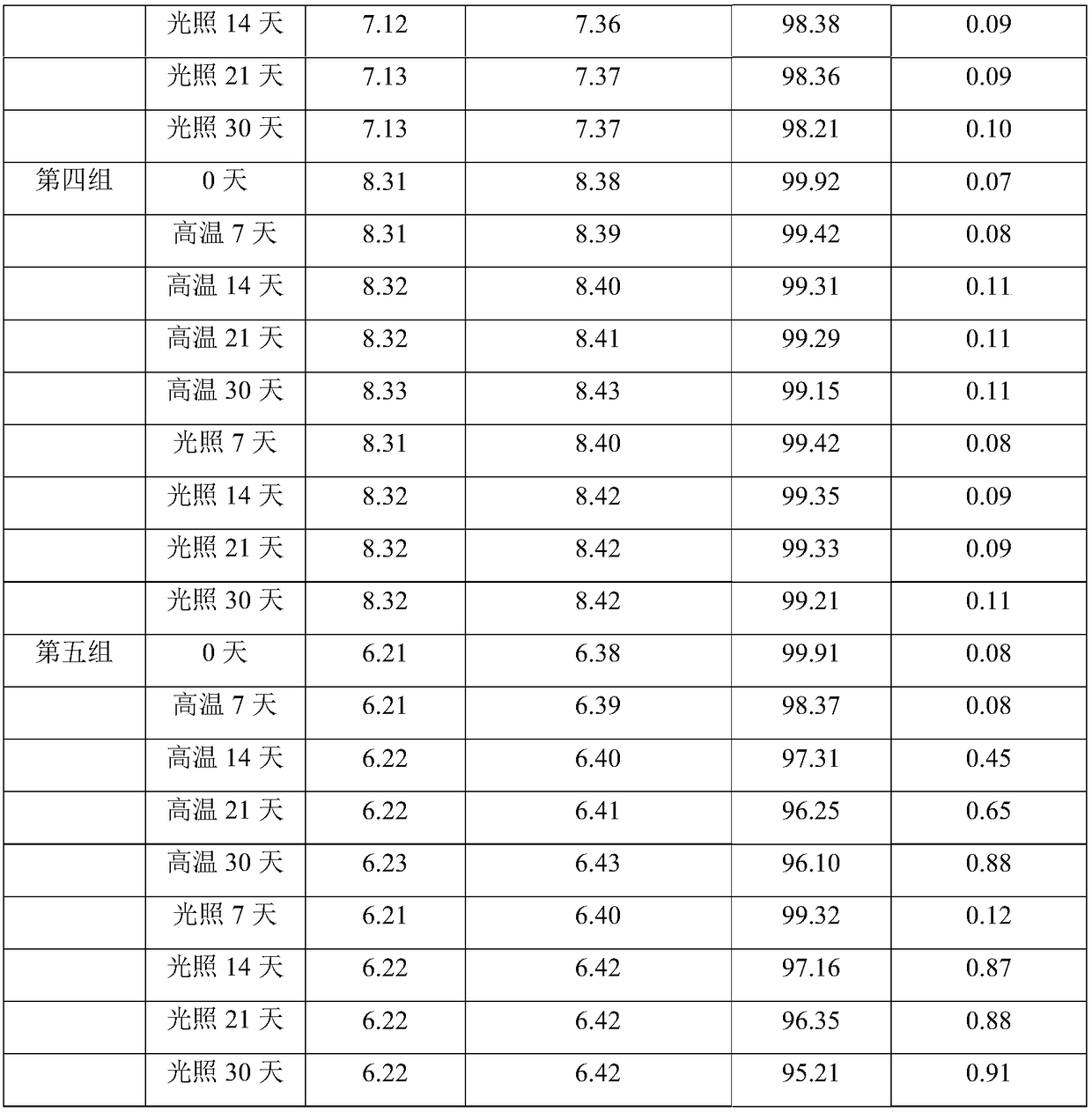
[0063] 1. Prepare a 0.005mol / L calcium hydrogen phosphate solution: anhydrous calcium hydrogen phosphate is dissolved in water for injection to a constant volume, and the pH is adjusted to 7.3 with phosphoric acid;
[0064] 2. Dissolve sodium chloride with 0.005mol / L calcium hydrogen phosphate solution and stir, then add cladribine and continue stirring. Sodium chloride and cladribine are mixed in a mass ratio of 8:1, and the pH value of the solution is detected , the dissolution temperature is controlled at 30°C;
[0065] 3. Check that the pH is greater than 7, adjust the pH to 7 with 0.5wt% phosphoric acid solution, and then add 0.005mol / L calcium hydrogen phosphate solution to continue to adjust the pH to above 7.0;
[0066] 4. Check that the pH is less than 7, add 0.005mol / L calcium hydrogen phosphate solution and continue to adjust the pH to above 7.0;
[0067] 5. Constant volume, packagin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com