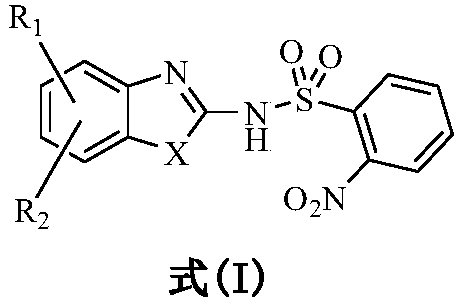
2-nitrophenylsulfonamide derivatives, and preparation method and medical application thereof
A nitro, pharmaceutical technology, applied in the field of 2-nitrophenylsulfonamide derivatives, can solve many problems such as
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-A
[0048] Synthesis of Example 1-A Compound 1 (Synthetic Method 1)
[0049]
[0050] Synthesis of Intermediate A
[0051] Dissolve 4-aminobenzonitrile (11.8g, 100mmol) in 100ml of acetic acid, add potassium thiocyanate (29.1g, 300mmol) and cool to about 10°C, slowly add liquid bromine (16g, 100mmol) dropwise, after the addition is complete Warm to room temperature and stir for 1 hour. The temperature was raised to 60° C., and the reaction was continued for 6 hours. Concentrate under reduced pressure, evaporate most of the acetic acid, add 100mL water, 200ml ethyl acetate, stir for 0.5 hours, filter with suction, wash with water, dry to obtain intermediate A 2-amino-6-cyanobenzothiazole (12.4g, yield 71%).
[0052] Synthesis of Compound 1 (Synthetic Method 1)
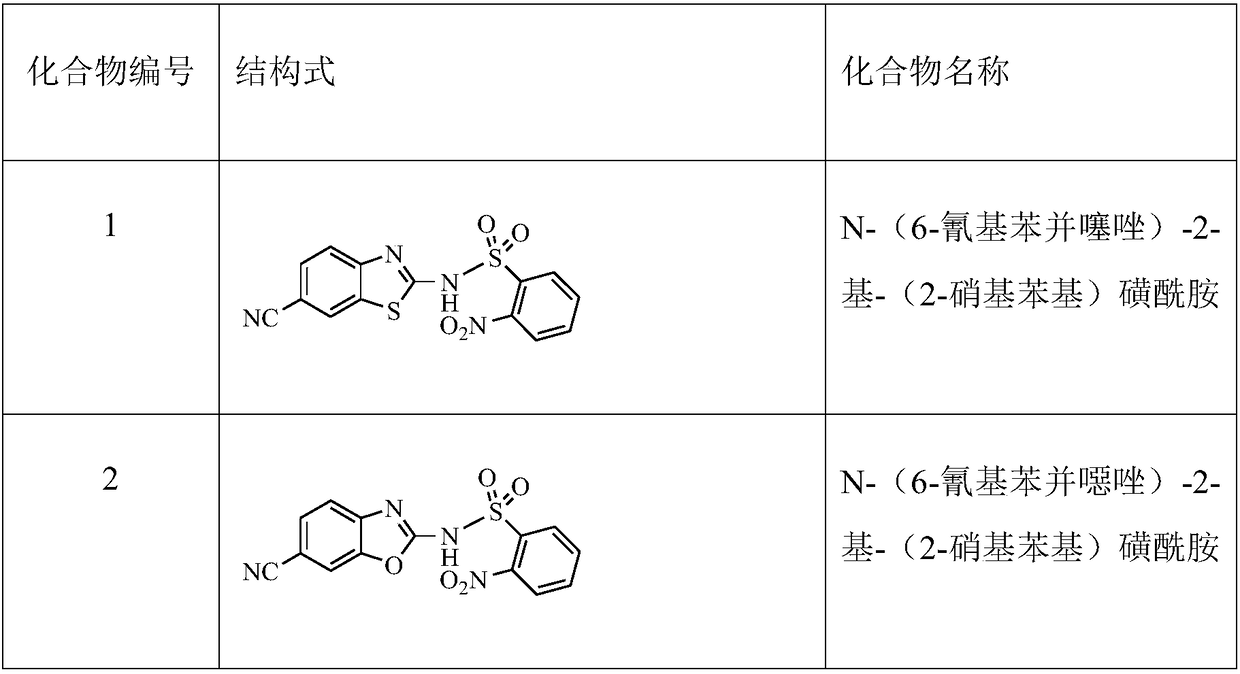
[0053] Dissolve compound A 2-amino-6-cyanobenzothiazole (700mg, 4mmol) in 10ml of pyridine, add 70mg of DMAP, cool to about 0°C with ice water, slowly add 2-nitrobenzene-1-sulfonyl chloride (1.06 g, 4.8mmol, 1.2eq),...
Embodiment 1-B
[0056] Synthesis of Example 1-B Compound 1 (Synthesis Method 2)
[0057]
[0058] Dissolve compound A 2-amino-6-cyanobenzothiazole (700mg, 4mmol) in 7ml toluene, add K 2 CO 3 (1.1g, 8mmol), 2-nitrobenzene-1-sulfonyl chloride (1.06g, 4.8mmol, 1.2eq), heated to reflux for about 10 hours after addition, to compound A 2-amino-6-cyanobenzo Thiazole disappears. Extracted with ethyl acetate, the crude product was separated and purified by silica gel column chromatography, the eluent was petroleum ether: ethyl acetate (V / V) = 1:2, to obtain compound 1 N-(6-cyanobenzothiazole)-2 -yl-(2-nitrophenyl)sulfonamide (1.12 g, yield 78%).
Embodiment 1-C
[0059] Synthesis of Example 1-C Compound 1 (Synthetic Method 3)
[0060]
[0061] Compound A 2-amino-6-cyanobenzothiazole (700mg, 4mmol) was dissolved in 7ml of anhydrous THF, cooled to about -20°C, and LDA / THF solution (4.8mL, 1mol / L, 1.2eq ), continue stirring at this temperature for 0.5 h after the addition, add 2-nitrobenzene-1-sulfonyl chloride (1.06 g, 4.8 mmol, 1.2 eq), and continue stirring at 0 ° C for 2 hours after the addition. Extracted with ethyl acetate, the crude product was separated and purified by silica gel column chromatography, the eluent was petroleum ether: ethyl acetate (V / V) = 1:2, to obtain compound 1N-(6-cyanobenzothiazole)-2- Amyl-(2-nitrophenyl)sulfonamide (1.22 g, yield 85%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com