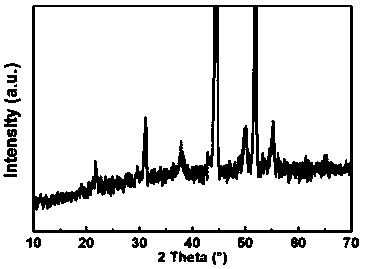
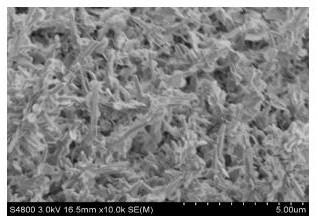
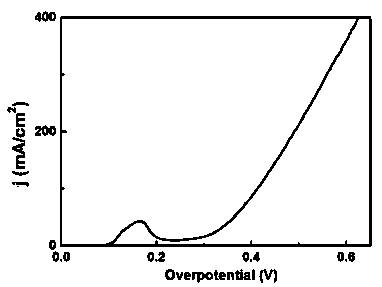
Nanometer forest-shaped V-doped Ni3S2/NF self supporting electrode and preparation method thereof
A self-supporting electrode, forest technology, applied in the direction of electrode, electrode shape/type, electrolysis process, etc., can solve the problems of not being able to perform at the same time, mismatch between hydrogen-producing end and oxygen-producing end, etc., to achieve short reaction time and improve conductivity and electron transfer efficiency, the effect of simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] (1) Immerse the conductive substrate in acetone solution for ultrasonic cleaning for 10 minutes, then transfer it to 2mol / L hydrochloric acid for ultrasonic cleaning for 10 minutes, and finally wash it alternately with ethanol and ultrapure water for 3 times, and then dry it in vacuum at 35°C for 10 hours;
[0018] (2) Configure a precursor solution, which contains nickel chloride at a concentration of 0.05mol / L, vanadium chloride at a concentration of 0.0125mol / L, ammonium fluoride at a concentration of 0.05mol / L and a concentration of 0.125 ) mol / L aqueous solution of urea, magnetically stirred at room temperature for 20 minutes to obtain a clear solution A. Transfer the clarified solution A and the conductive substrate treated in step (1) into a high-temperature and high-pressure hydrothermal kettle, and then react at 100°C for 18 hours, and the reaction filling ratio should be controlled at 40%. After the hydrothermal reaction was completed, the reactor was naturall...
Embodiment 2
[0021] (1) Soak nickel foam of 1cm x 5cm in acetone solution for 10 minutes, then transfer it to 2mol / L hydrochloric acid for 10 minutes, and finally rinse it with ethanol and ultrapure water for 3 times, and then clean it at 35°C. Vacuum drying for 10h;
[0022] (2) Configure a precursor solution, which contains nickel chloride at a concentration of 0.1mol / L, vanadium chloride at a concentration of 0.04mol / L, ammonium fluoride at a concentration of 0.05mol / L and a concentration of 0.2 The aqueous solution of mol / L urea was magnetically stirred at room temperature for 20 minutes to obtain a clear solution A. Transfer the clarified solution A and the conductive substrate treated in step (1) into a high-temperature and high-pressure hydrothermal kettle, and then react at 120°C for 14 hours, and the reaction filling ratio should be controlled at 40%. After the hydrothermal reaction was completed, the reactor was naturally cooled to room temperature, and then the conductive subst...
Embodiment 3
[0025] (1) Sonicate 1cm x 5cm nickel foam in acetone solution for 5 minutes, then immerse foam nickel in 2mol / L hydrochloric acid for 5 minutes, and finally rinse with ethanol and ultrapure water for 3 times respectively. After vacuum drying for 10°C, the treated foamed nickel was obtained;
[0026] (2) Configure a precursor solution, which contains nickel chloride at a concentration of 0.1mol / L, vanadium chloride at a concentration of 0.05mol / L, ammonium fluoride at a concentration of 0.05mol / L and 0.2 The aqueous solution of mol / L urea was magnetically stirred at room temperature for 20 minutes to obtain a clear solution A. Transfer the clarified solution A and the nickel foam treated in step (1) into a high-temperature and high-pressure hydrothermal kettle, and then react at 140°C for 10 hours, and the reaction filling ratio should be controlled at 30%. After the hydrothermal reaction was completed, the reactor was naturally cooled to room temperature, and then the conduct...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com