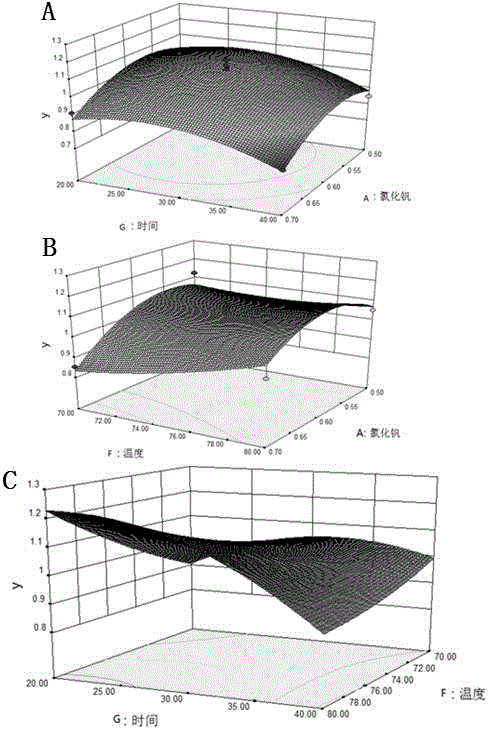
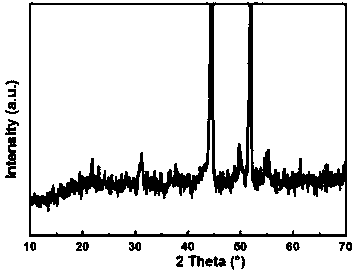
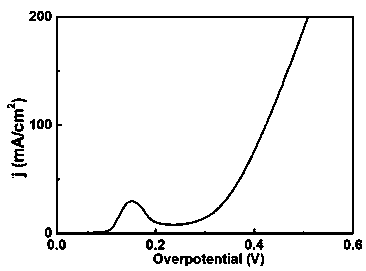
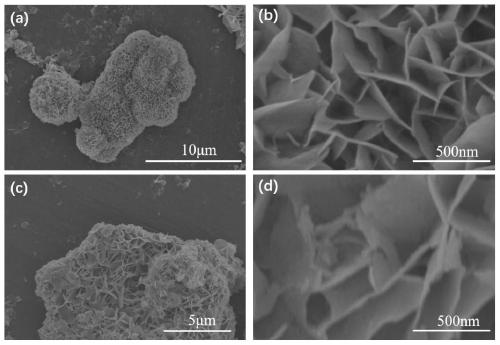
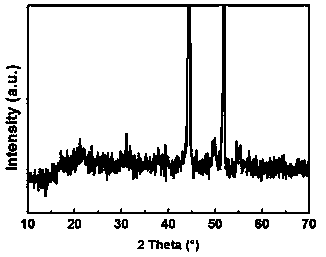
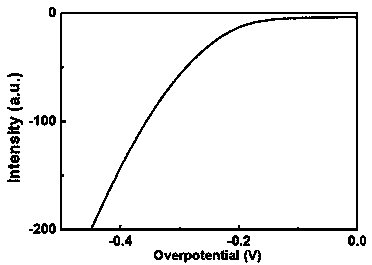
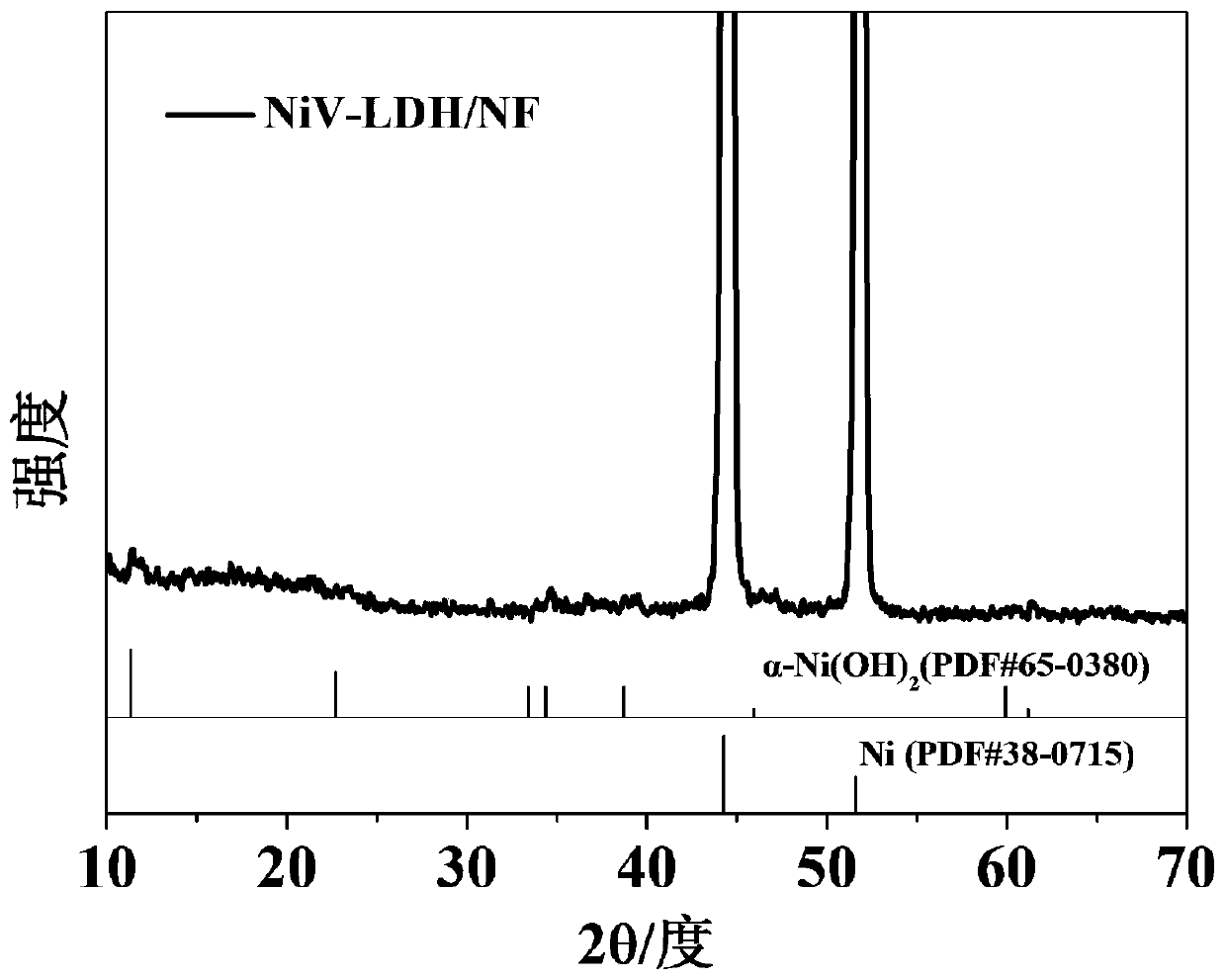
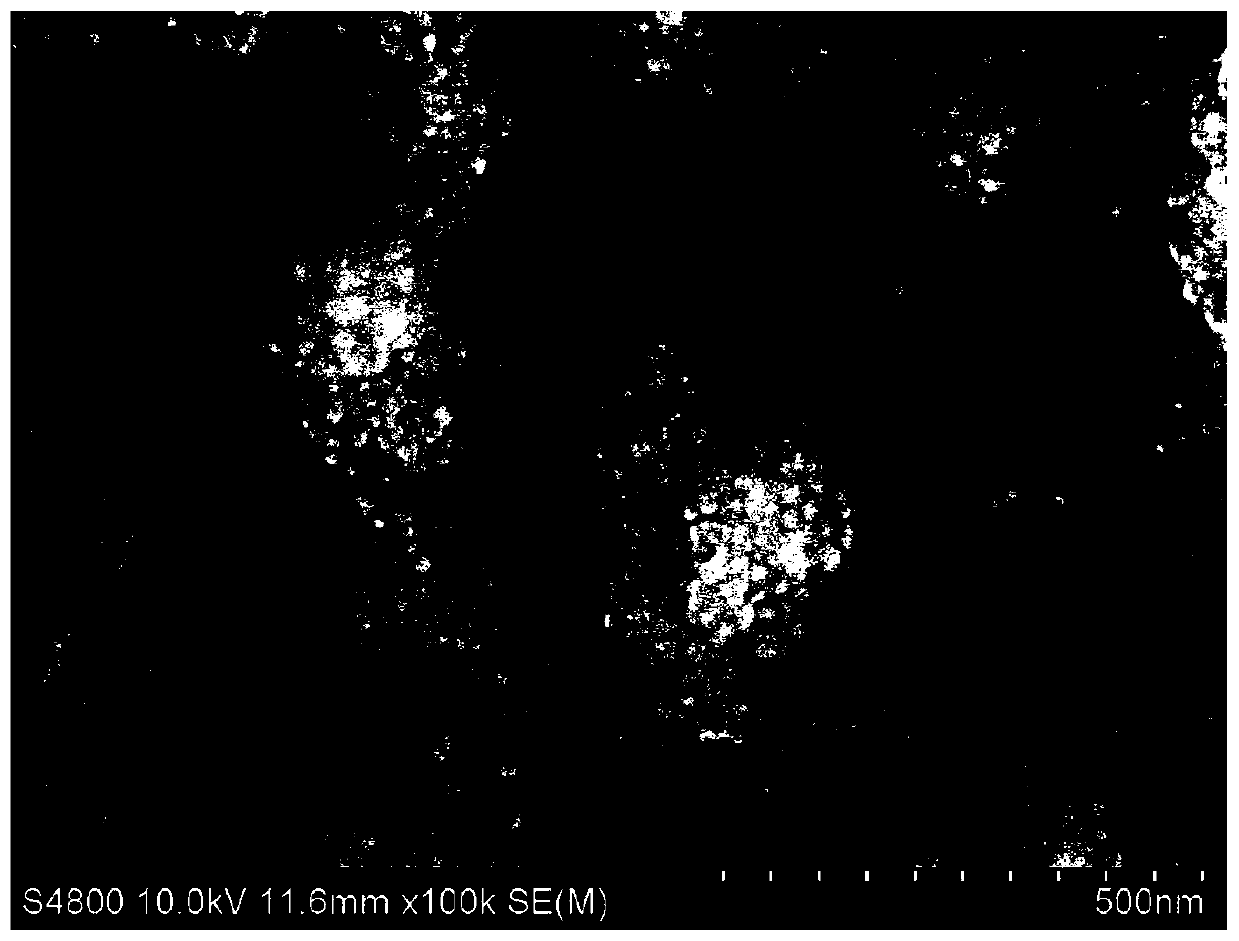
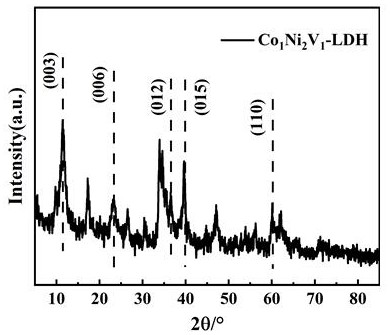
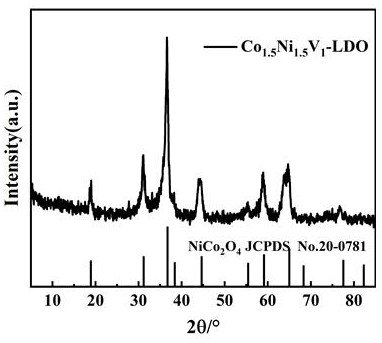
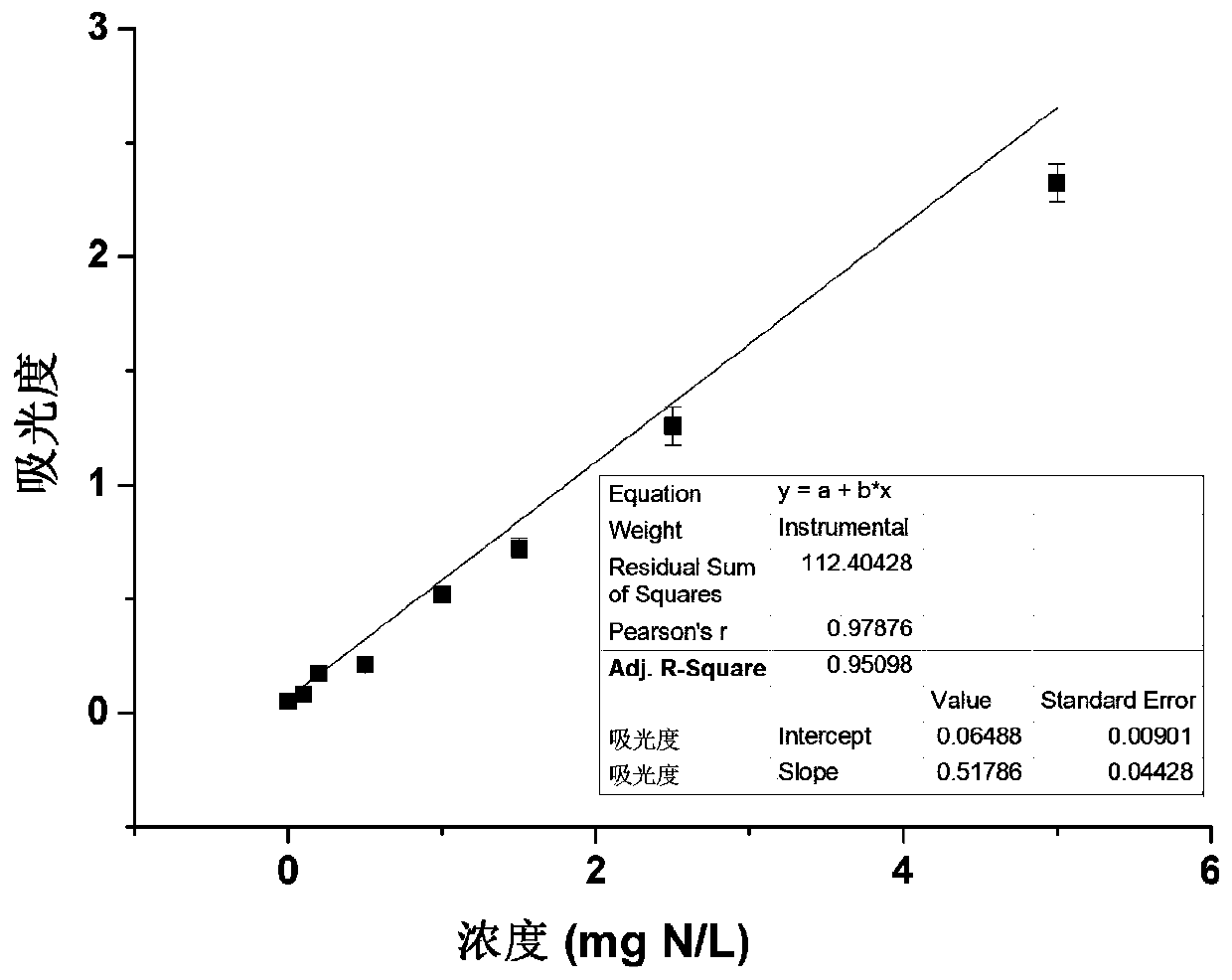
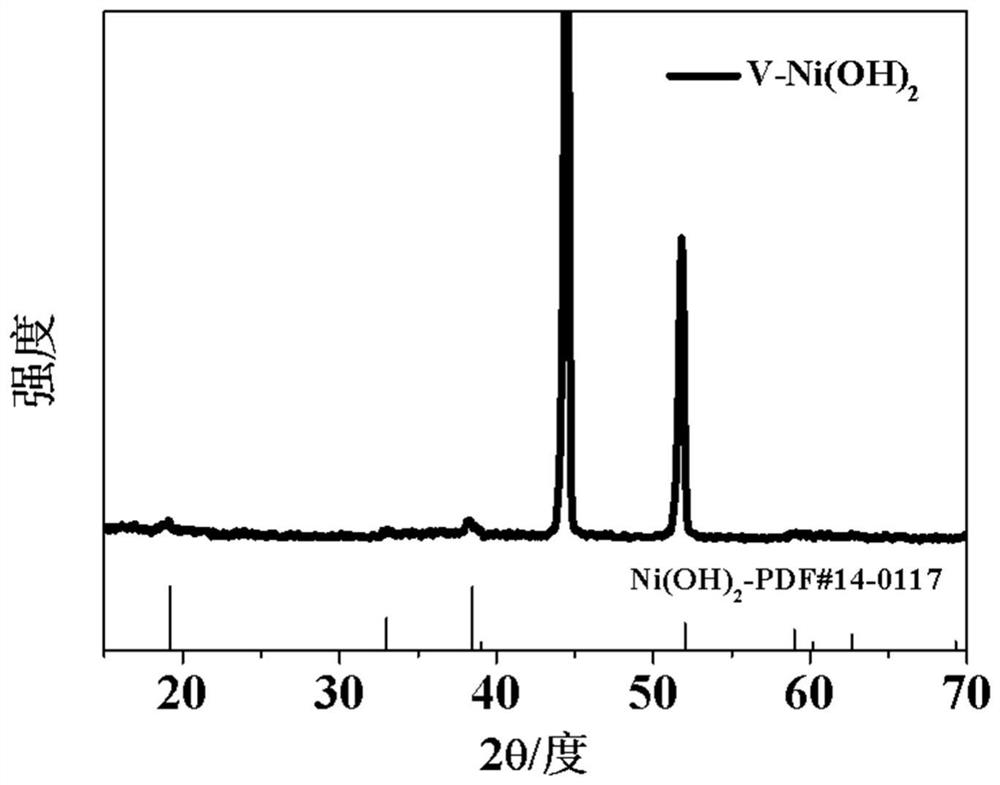
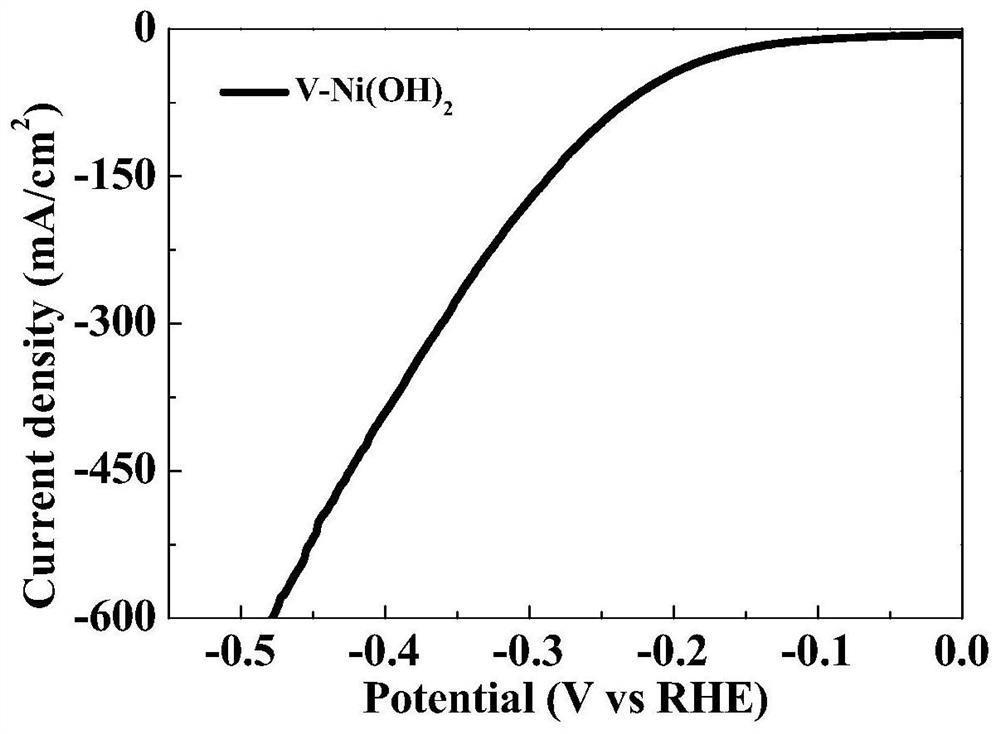
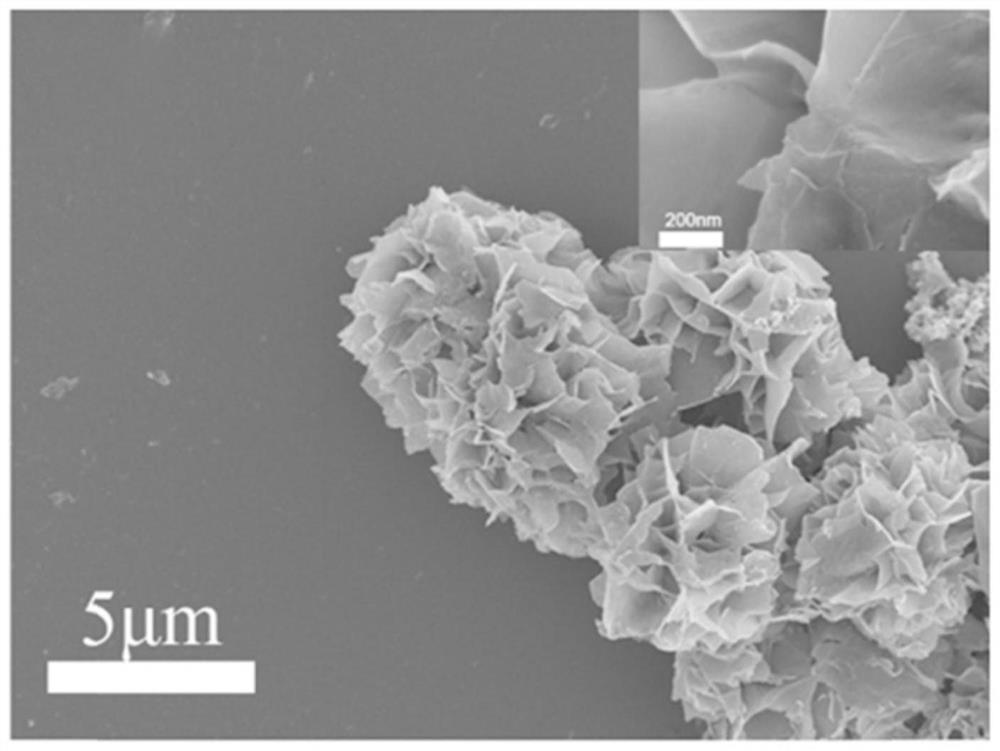
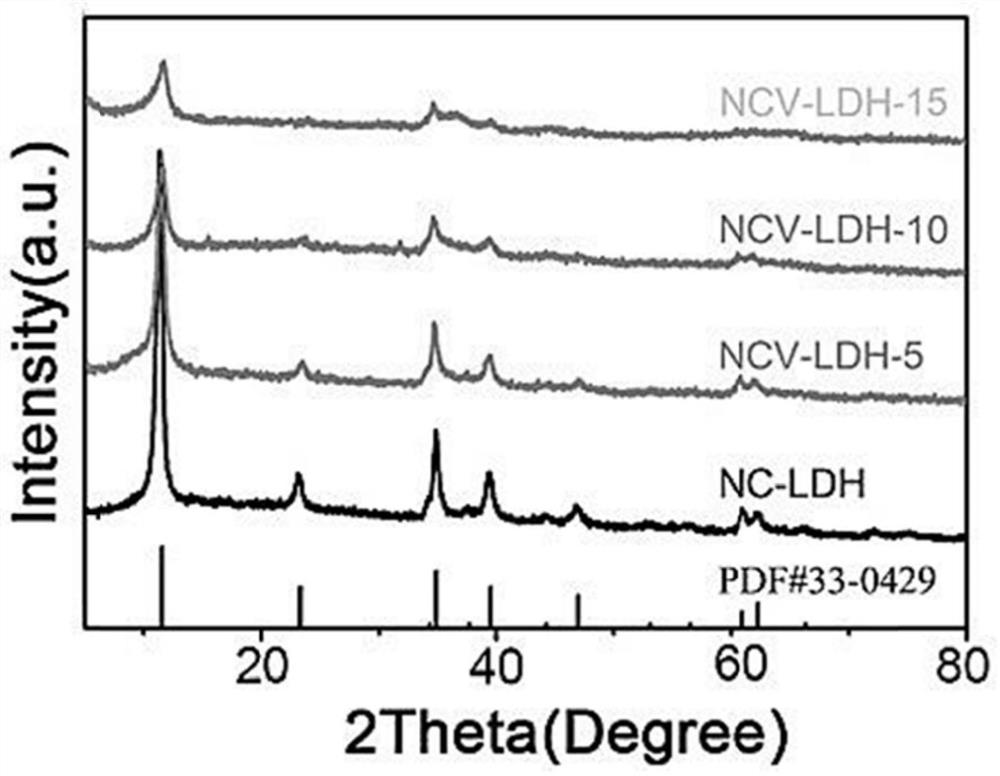
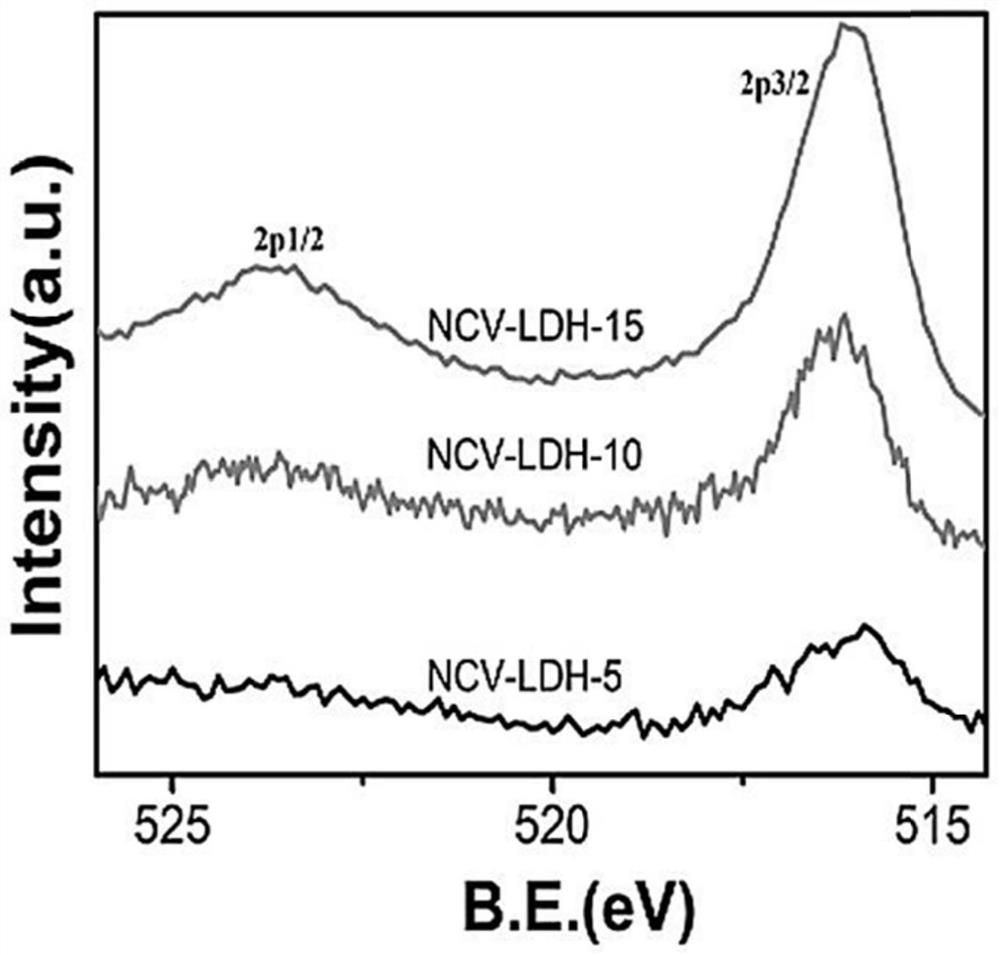
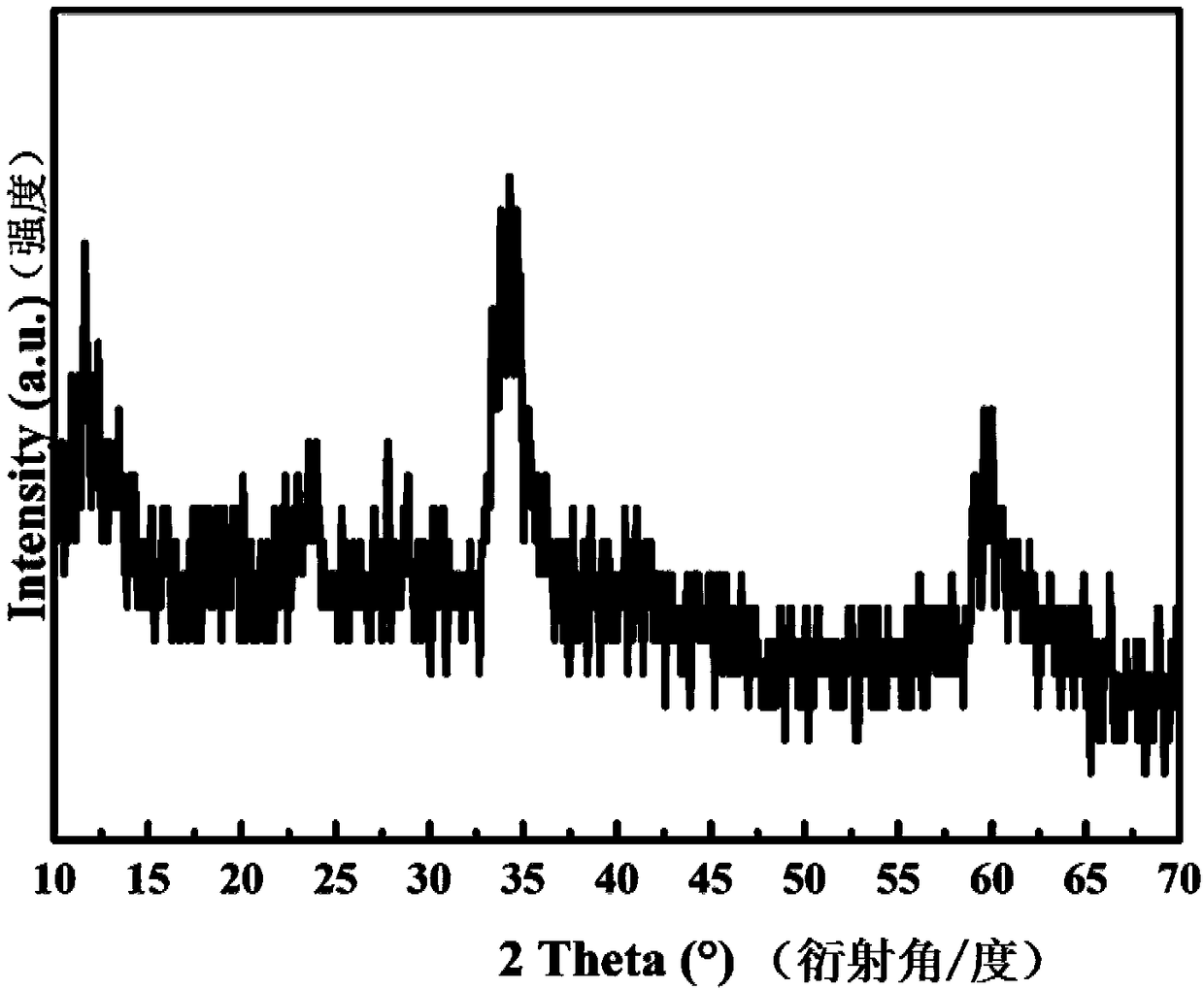
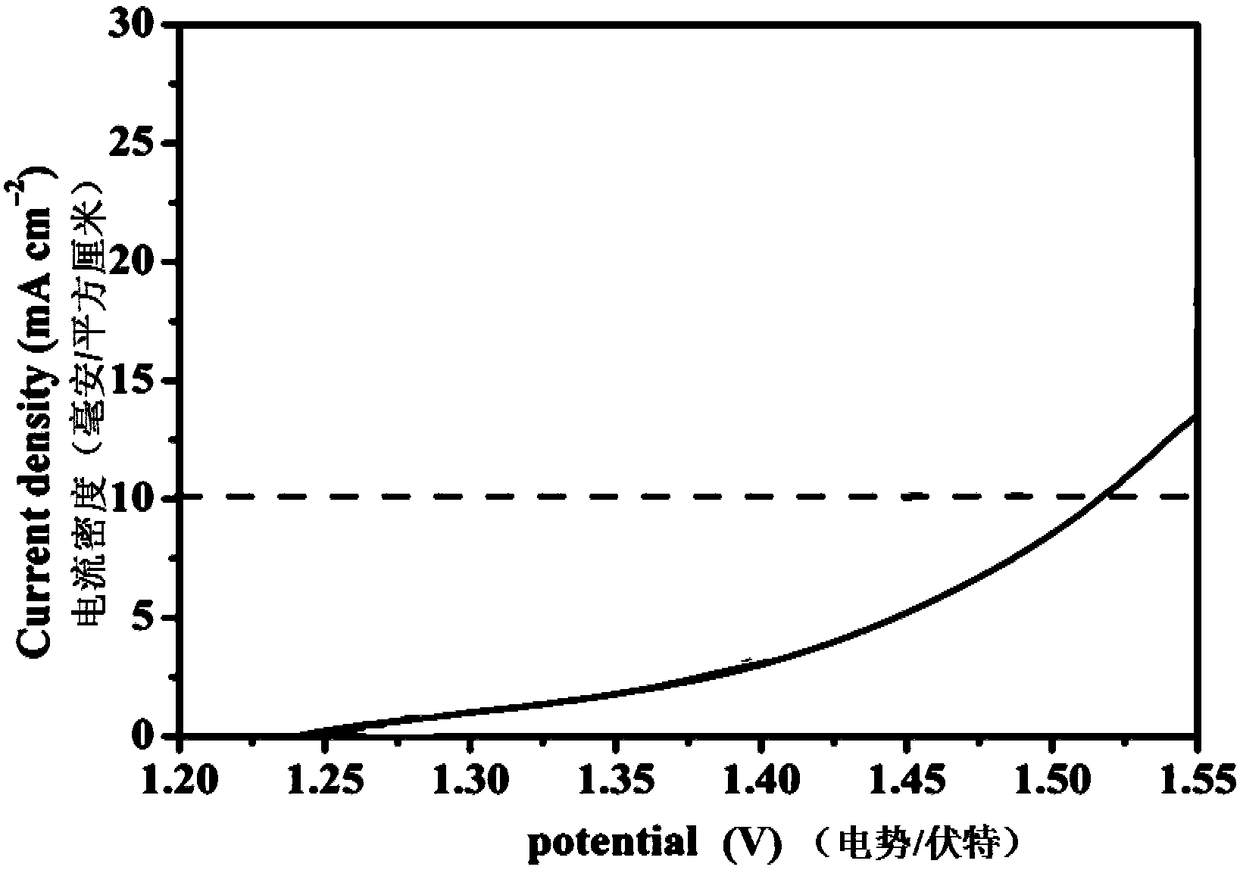
Patents
Literature
70 results about "Vanadium chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vanadium chloride may refer to: Vanadium chloride Vanadium chloride Vanadium chloride
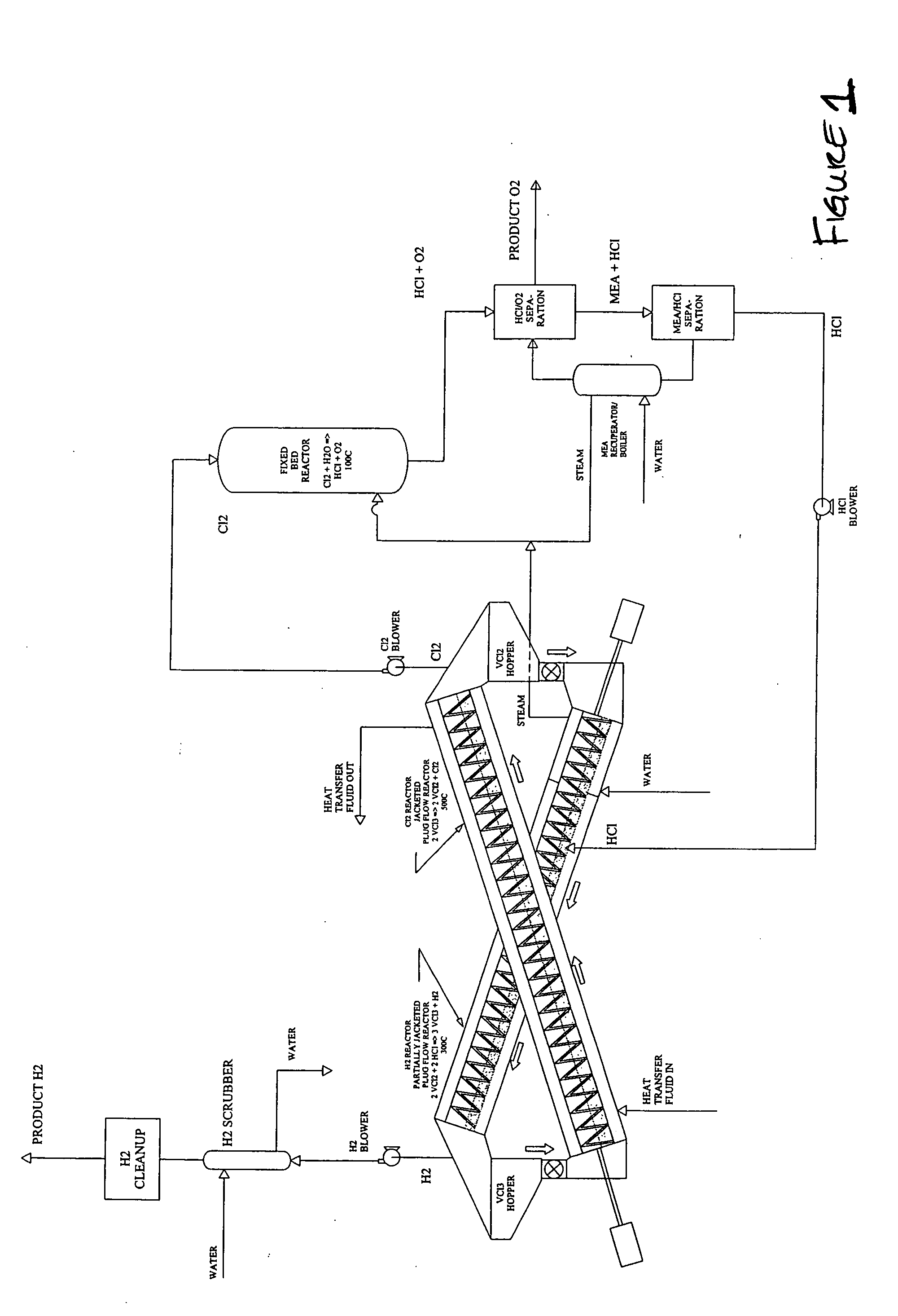
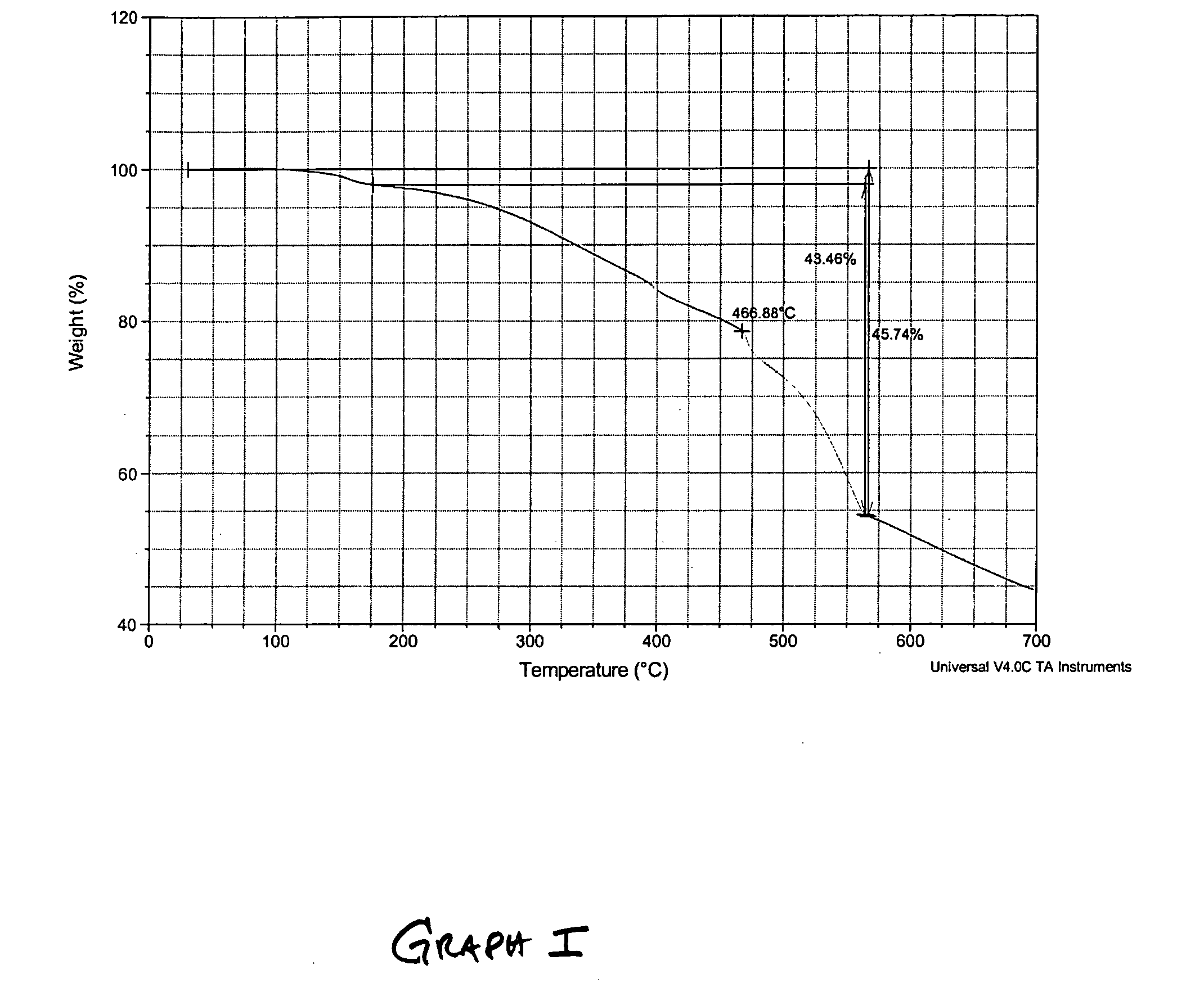
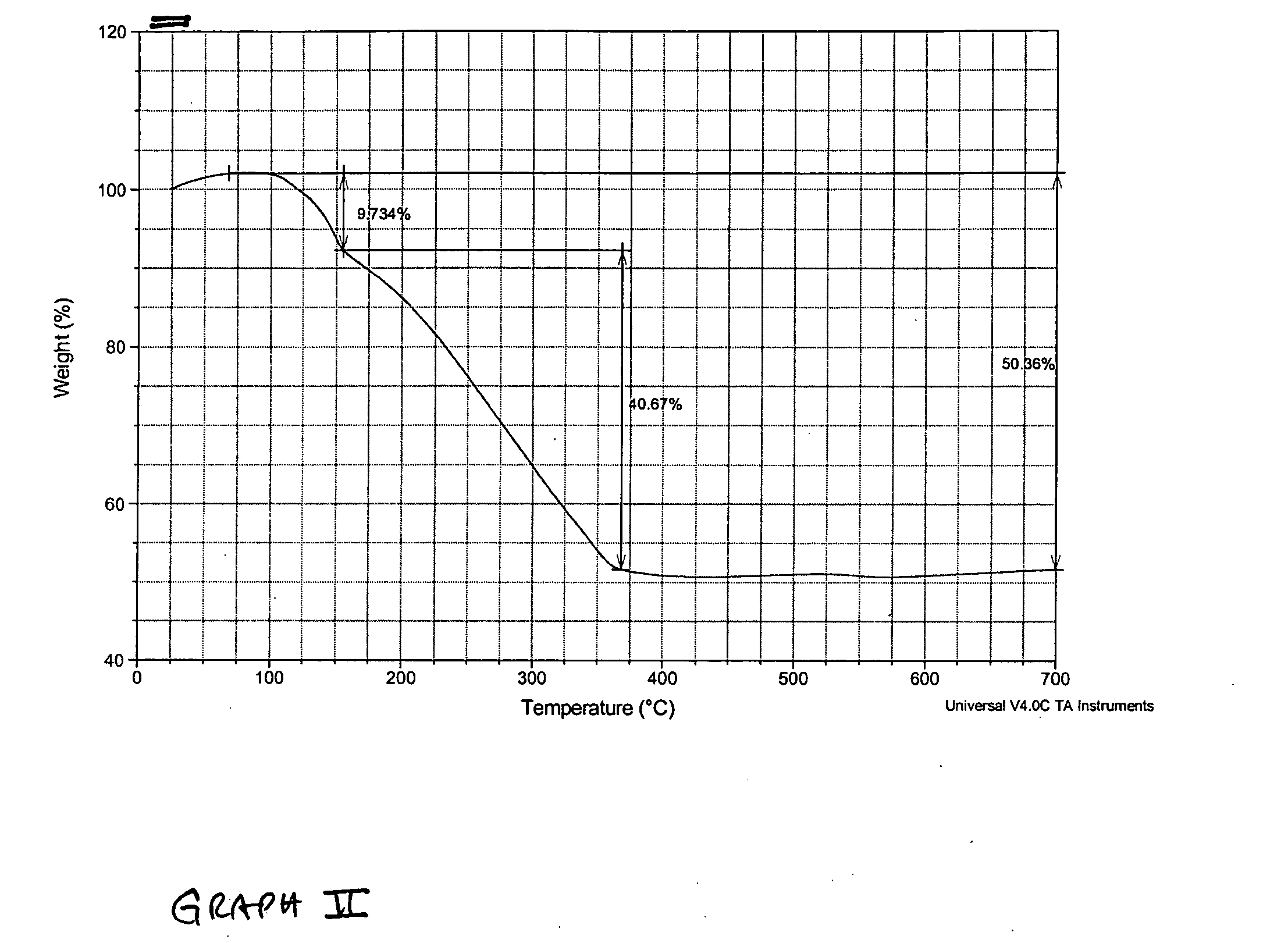
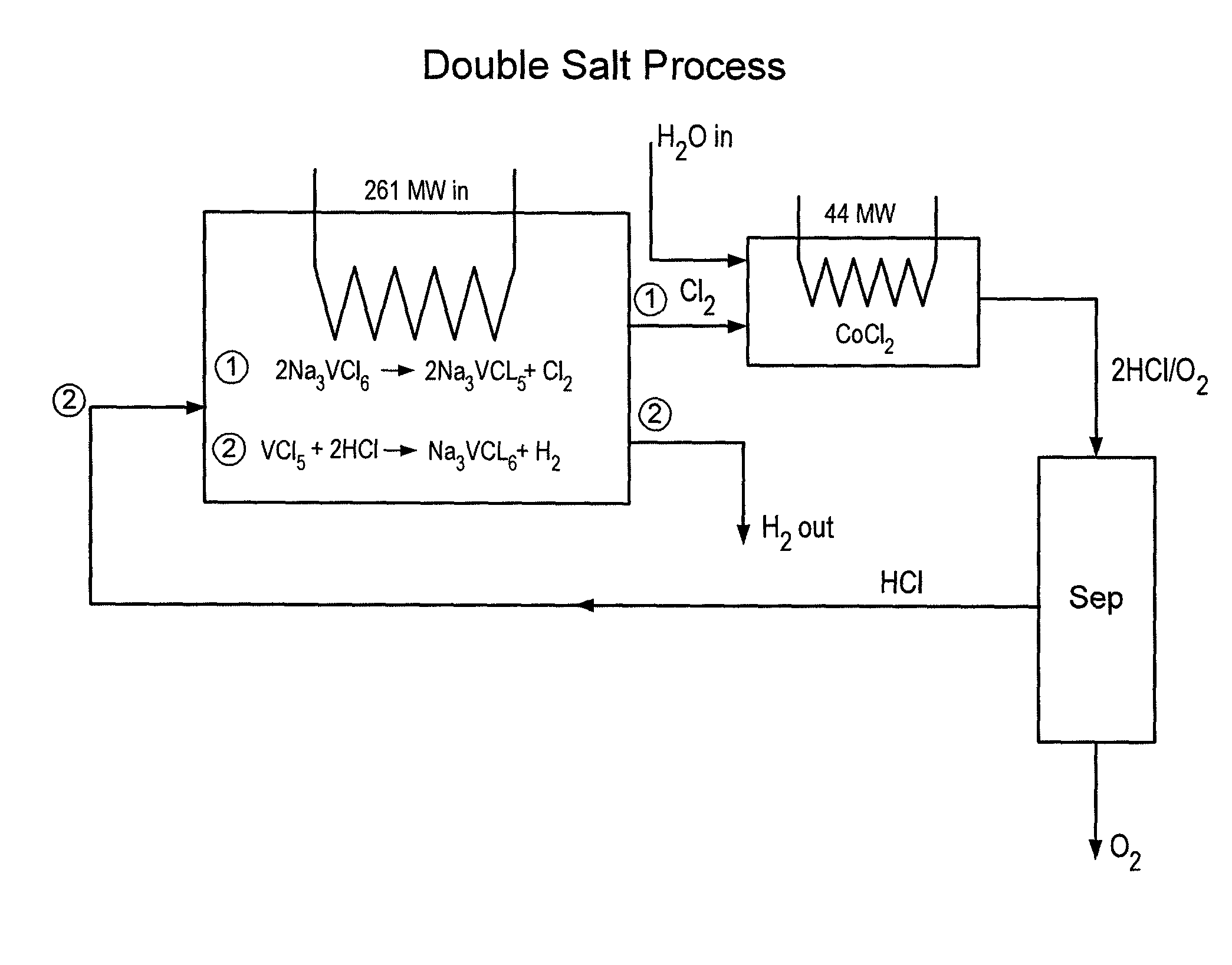
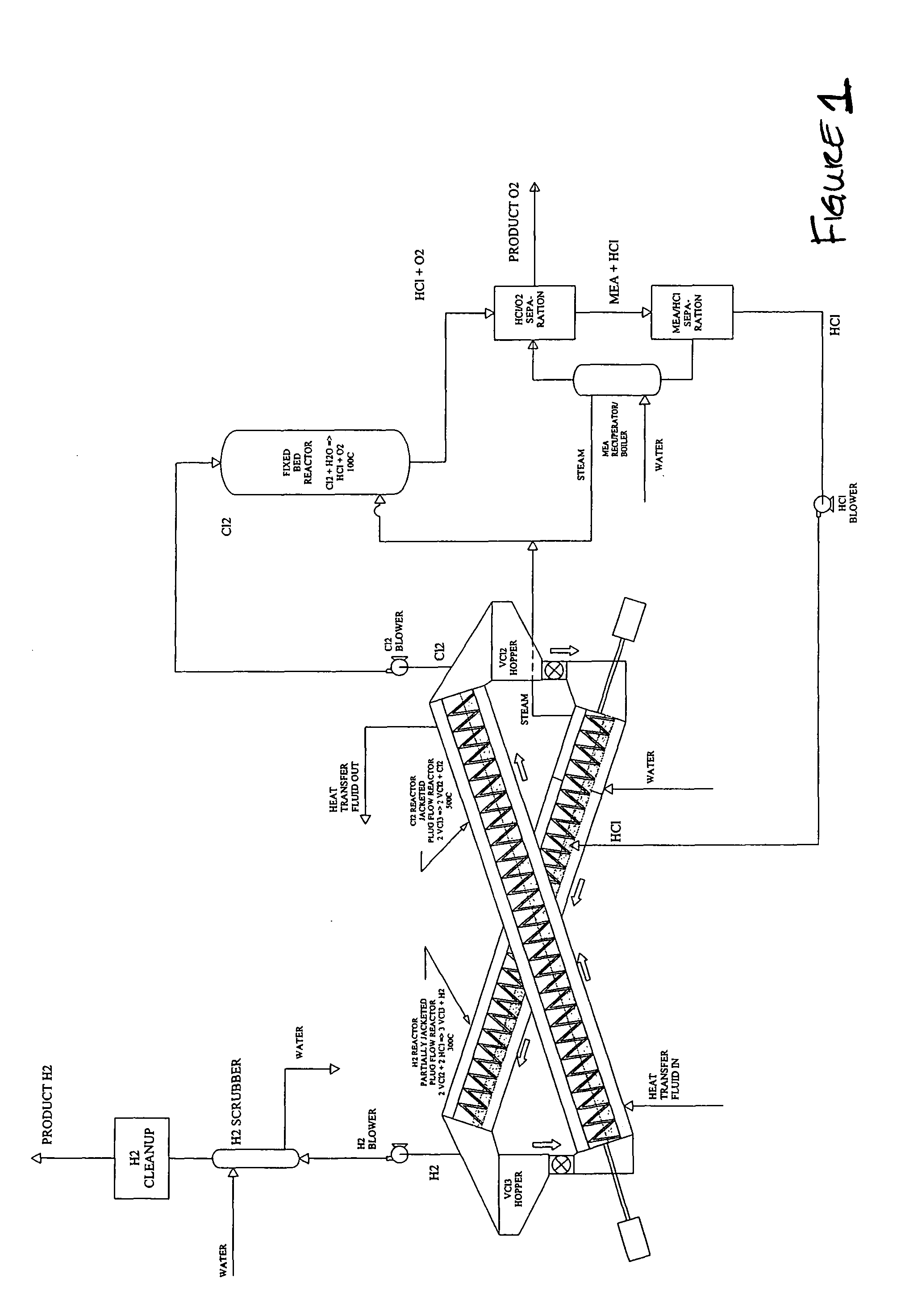
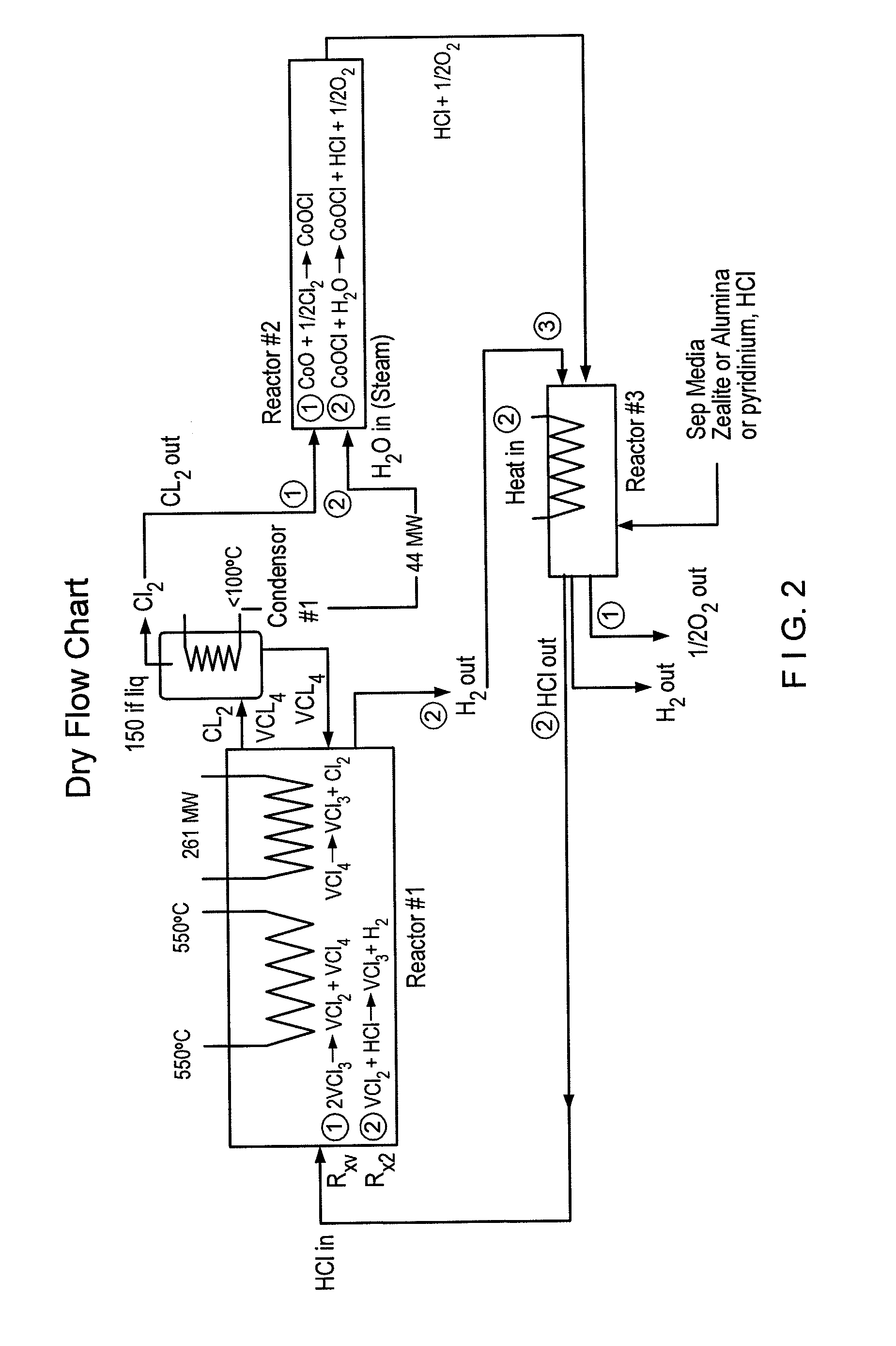
Thermochemical hydrogen produced from a vanadium decomposition cycle
InactiveUS20050013771A1Improve reliabilityHydrogen separation using liquid contactEnergy inputHydrogenDecomposition
A thermochemical water-splitting process all reactions of which operate at relatively low temperatures and high efficiencies, and in which relatively inexpensive materials and processing methods are made possible. This invention involves the decomposition of a metal halide compound, i.e., one which is capable of being reduced from a higher oxidation state to lower oxidation state, e.g. vanadium chloride III→vanadium dichloride. The process is cyclic and regenerative, and the only net inputs are water and heat; and the only net outputs are hydrogen and oxygen. The process makes it possible to utilize a wide variety of available heat, including solar, sources for the energy input.
Owner:AMENDOLA STEVEN
Thermochemical hydrogen produced from a vanadium decomposition cycle
InactiveUS7799315B2Improve reliabilityHigh thermal efficiencyHydrogen separation using liquid contactEnergy inputHydrogenDecomposition
A thermochemical water-splitting process all reactions of which operate at relatively low temperatures and high efficiencies, and in which relatively inexpensive materials and processing methods are made possible. This invention involves the decomposition of a metal halide compound, i.e., one which is capable of being reduced from a higher oxidation state to lower oxidation state, e.g. vanadium chloride III→vanadium dichloride. The process is cyclic and regenerative, and the only net inputs are water and heat; and the only net outputs are hydrogen and oxygen. The process makes it possible to utilize a wide variety of available heat, including solar, sources for the energy input.
Owner:AMENDOLA STEVEN
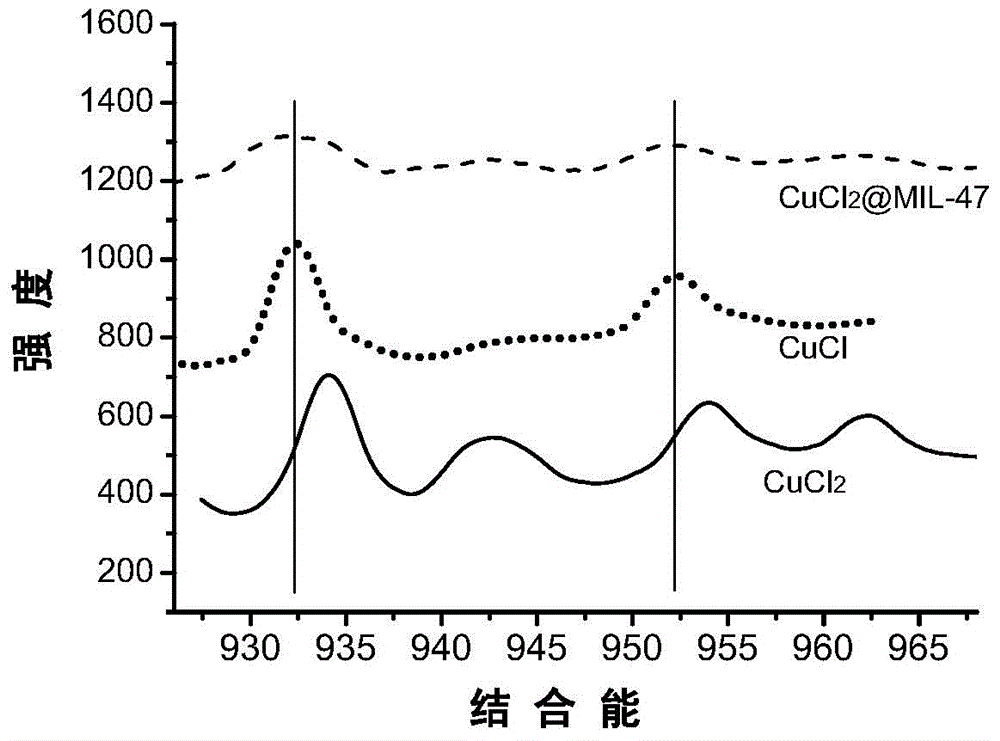
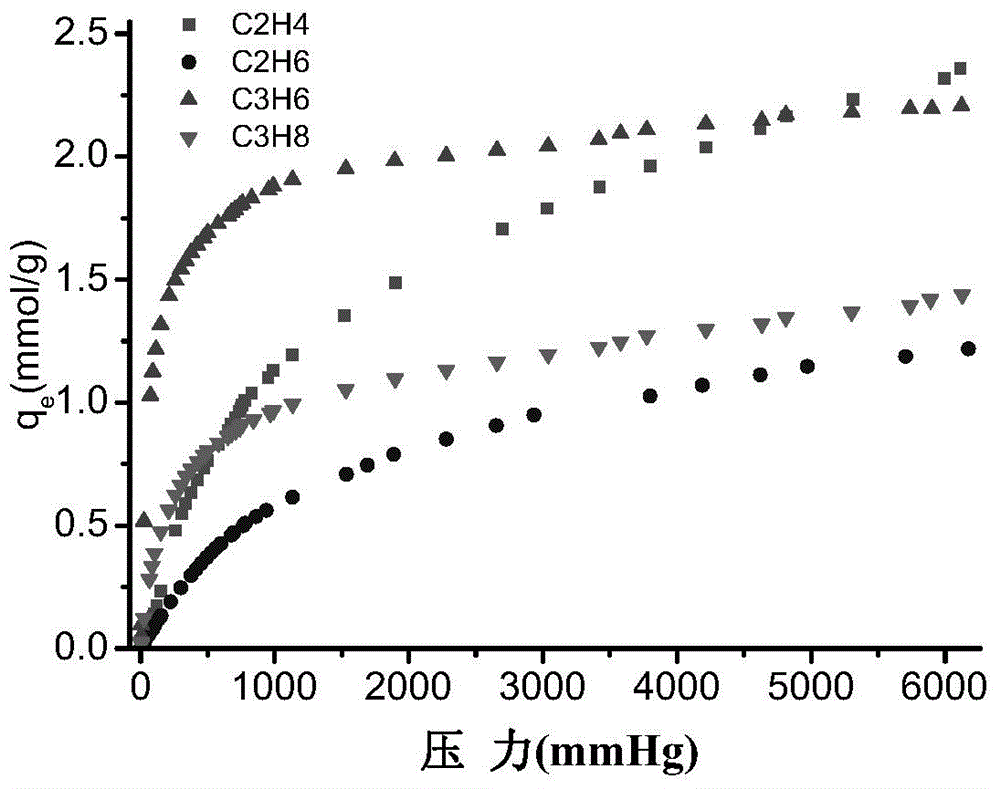
Adsorbent for olefin/alkane mixed gas separation and preparation method and application thereof
ActiveCN104525121AInhibitory concentrationAvoid the problem of being easily oxidizedOther chemical processesAdsorption purification/separationAlkaneSorbent
The invention discloses a preparation method of an adsorbent for olefin / alkane mixed gas separation. the preparation method comprises the following steps: after vanadium chloride, terephthalic acid, hydrofluoric acid and water are mixed, a hydrothermal reaction is carried out to obtain MIL-47(V<3+>) containing impurities; under the assistance of ultrasonic wave, low temperature activation treatment is carried out to remove impurities so as to obtain MIL-47(V<3+>); and by a solution impregnation method, Cu<2+> is loaded on MIL-47(V<3+>), and autoxidation-reduction is carried out to reduce the loaded Cu<2+> to Cu<+> so as to obtain the adsorbent. By using MIL-47(V<3+>) obtained by low temperature activation treatment as a carrier, Cu<2+> is firstly loaded, and then the loaded Cu<2+> is reduced to Cu<+> by the process of autoxidation-reduction. The preparation process is simple, and conditions are mild. The prepared adsorbent can be used to realize high-selective separation of olefin / alkane mixed gases.
Owner:ZHEJIANG UNIV
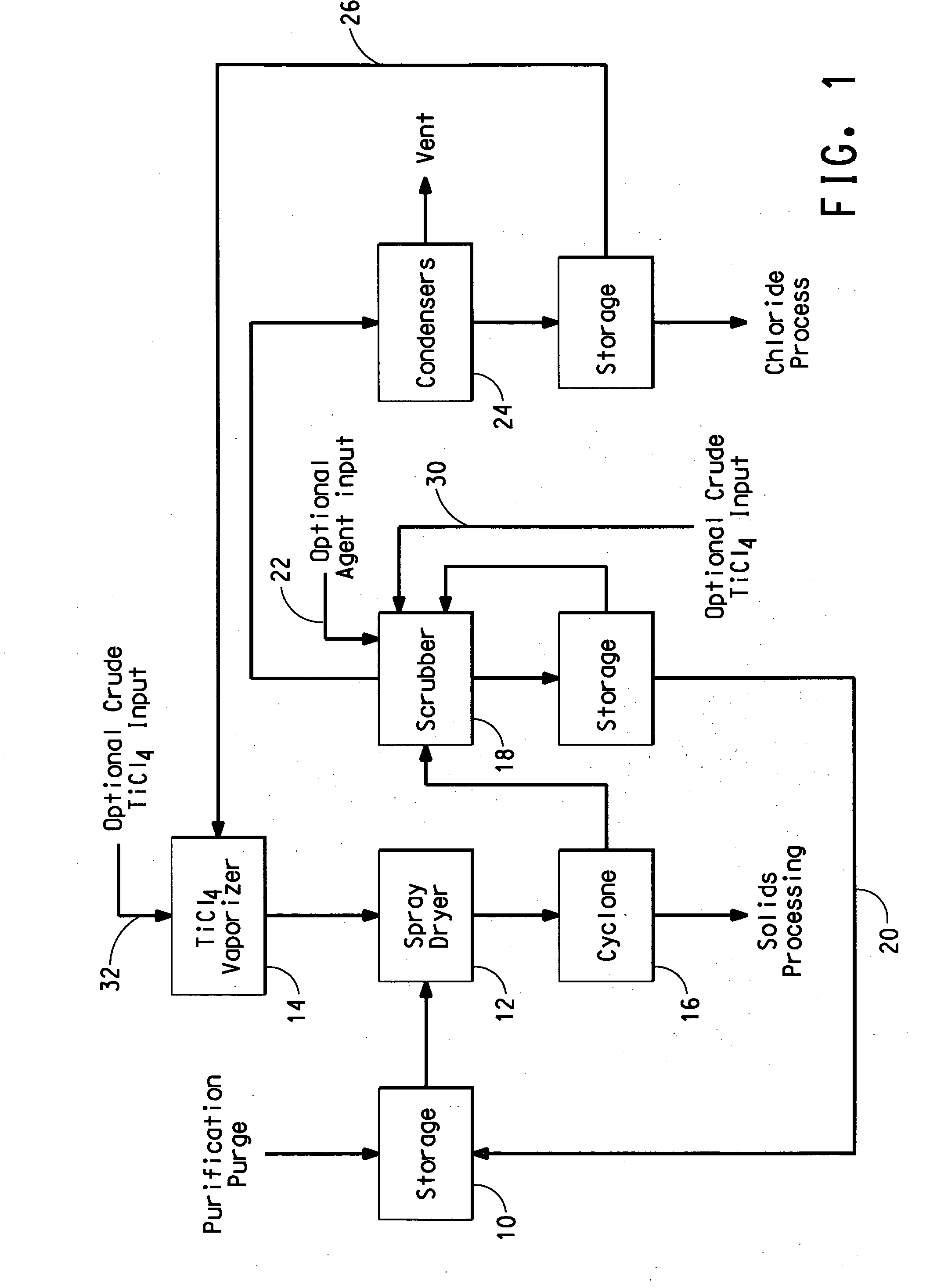
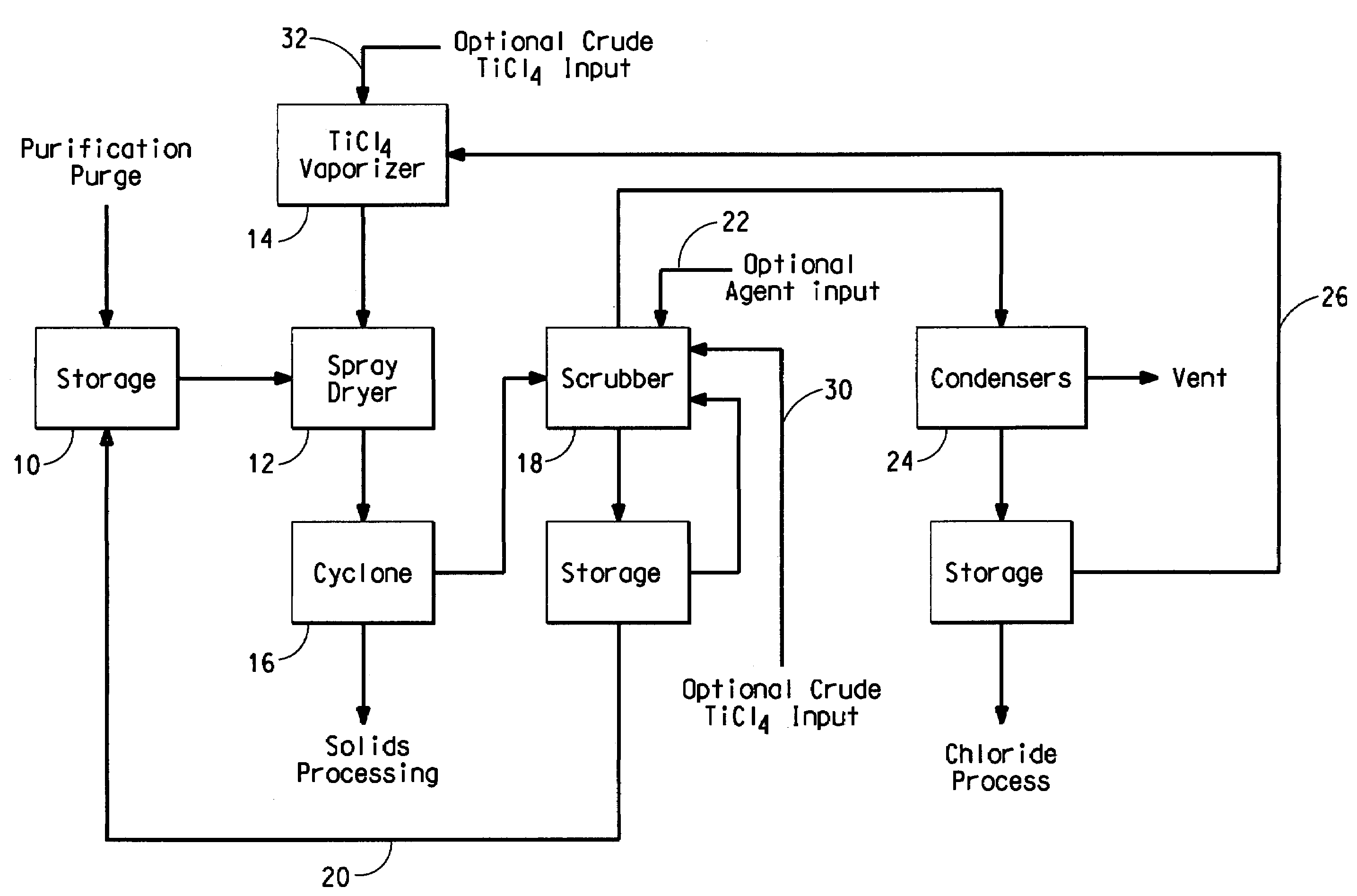
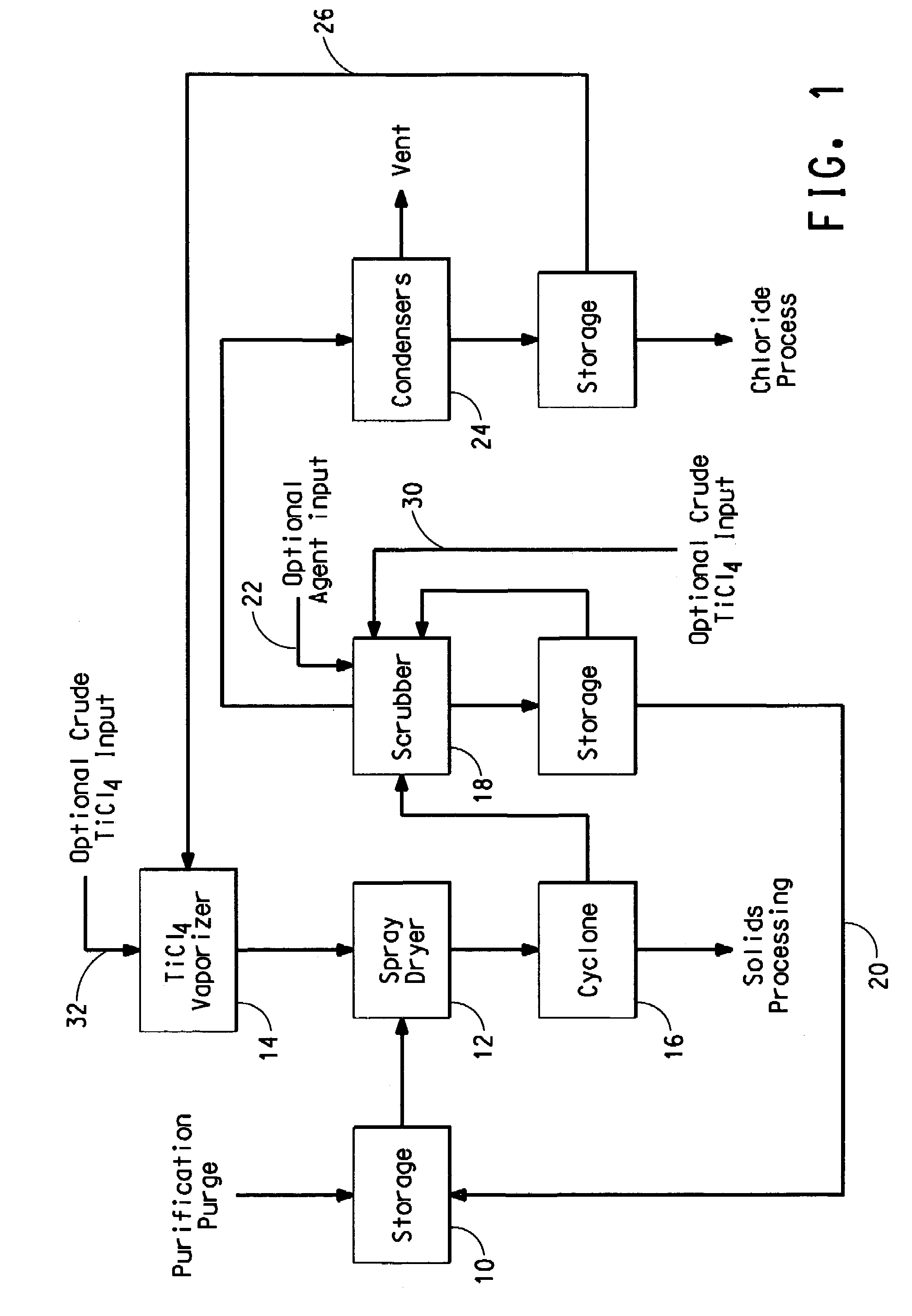
Process for separating solids from a purification purge stream
The disclosure is directed to a process for isolating solids from a purification purge stream comprising an impurity present as a solid, wherein the purification purge stream is substantially free of chlorides other than titanium tetrachloride and vanadium chloride, the process comprising the steps of: (a) atomizing the purification purge stream comprising titanium tetrachloride as a liquid and an impurity present as a solid; (b) drying solids in the atomized purification purge stream by contacting the atomized stream with a titanium tetrachloride vapor stream such that the combined streams reach a temperature of at least about 140° C. to vaporize the liquid titanium tetrachloride, wherein the titanium tetrachloride vapor is substantially free of chlorides other than those of titanium and vanadium, and substantially free of non-condensable gases comprising CO, CO2, N2, or mixtures thereof; and (c) separating the impurity present as a solid from the vaporized titanium tetrachloride. The separated vanadium solids may be further processed to recover valuable by products.
Owner:THE CHEMOURS CO TT LLC
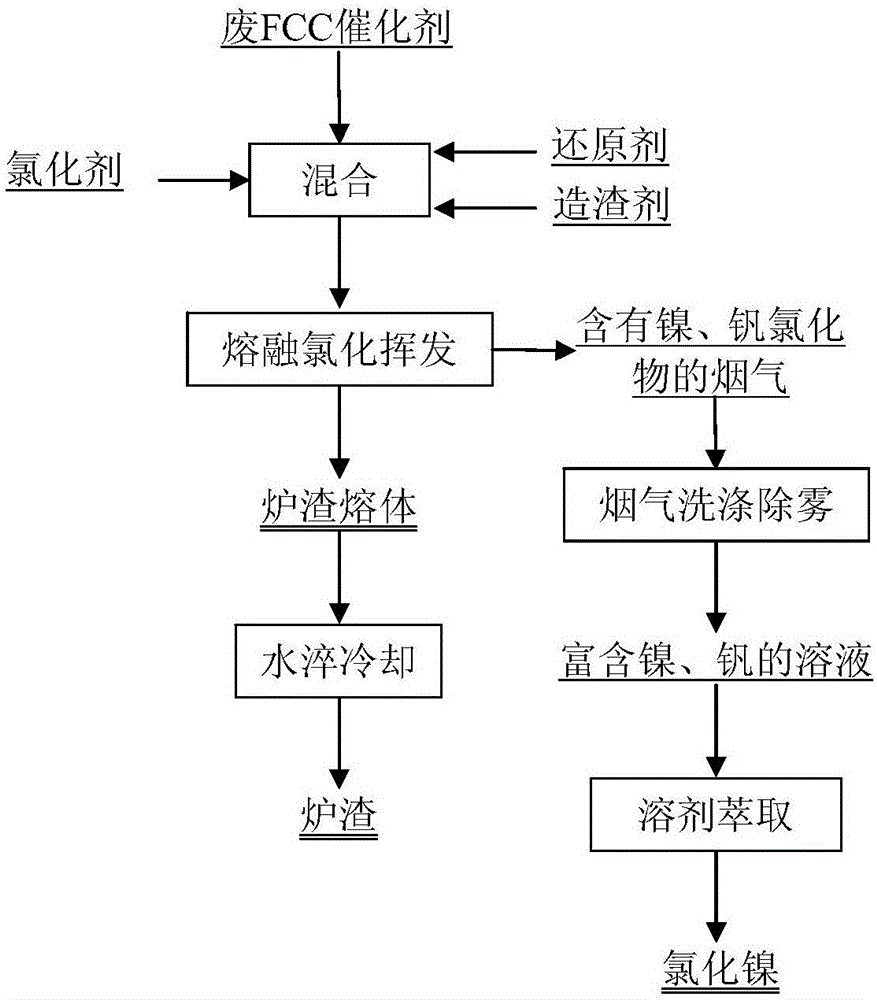
Method for recovering nickel and vanadium from waste FCC (Fluid Catalytic Cracking) catalyst through molten chlorination volatilizing
The invention discloses a method for recovering nickel and vanadium from a waste FCC (Fluid Catalytic Cracking) catalyst through molten chlorination volatilizing. The method comprises the following steps: adding a chlorinating agent, a reducing agent and a slag forming constituent to the waste FCC catalyst, and uniformly mixing to obtain a mixture to be molten; performing molten chlorination on the mixture to be molten through a melting furnace for 30 to 300 minutes at the temperature of 1250 to 1700 DEG C so as to obtain smoke containing nickel and vanadium chloride; charging the smoke containing the nickel and vanadium chloride into a smoke washing system to wash and defog the smoke so as to obtain a solution rich in nickel and vanadium; and then separating nickel and vanadium from the solution rich in nickel and vanadium by a solvent extraction method so as to obtain vanadium pentoxide and nickel chloride. With the adoption of the method, the recovery rate of nickel and vanadium can be greatly increased; in addition, the method is simple in technology, short in process, flexible to operate, high in recycling rate, and high in geographical adaptability; the produced wastewater, waste gas and slag are nontoxic and harmless, so that the environment is influenced a little.
Owner:BEIJING GENERAL RES INST OF MINING & METALLURGY
Method for removing vanadium impurity in titanic chloride by forced assisted-circulation heating
InactiveCN101423247APrevent volatilizationAvoid hydrolysisChemical industryTitanium halidesSludgeHigh pressure
The invention provides a method for removing impurity vanadium in titanium tetrachloride by forced external circulation heating, which comprises the following steps: coarse titanium tetrachloride enters an evaporator for vanadium removing reaction from a coarse titanium tetrachloride storage tank, reacts with mineral oil from a mineral oil storage tank to generate low valance vanadium chloride like vanadium oxychloride and the like and carbon, and is transported to a forced circulation heat interchanger through a submerged sludge pump by pressurizing, titanium tetrachloride passes through a tube pass, while heating medium passed through a shell pass; titanium tetrachloride flows at high speed under high pressure in the tube pass, flows out of the heat interchanger, and is sprayed into the evaporator for vanadium removing reaction, afterward, a part of the titanium tetrachloride is gasified and led into a vanadium removing tower, while ferric trichloride in the coarse titanium tetrachloride, low valance vanadium chloride like vanadium oxychloride and the like and carbon generated by reaction remains in the evaporator for vanadium removing reaction in a suspended state. As forced external circulation heating is adopted, titanium tetrachloride flows at high speed under high pressure, which prevents suspended substances from depositing and scarring on the wall of the heating tube, improves the heating efficiency by 20 percent-30 percent, and prolongs the service life of the heat interchanger.
Owner:锦州金业化冶技术有限公司
Process for separating solids from a purification purge stream
The disclosure is directed to a process for isolating solids from a purification purge stream comprising an impurity present as a solid, wherein the purification purge stream is substantially free of chlorides other than titanium tetrachloride and vanadium chloride, the process comprising the steps of: (a) atomizing the purification purge stream comprising titanium tetrachloride as a liquid and an impurity present as a solid; (b) drying solids in the atomized purification purge stream by contacting the atomized stream with a titanium tetrachloride vapor stream such that the combined streams reach a temperature of at least about 140° C. to vaporize the liquid titanium tetrachloride, wherein the titanium tetrachloride vapor is substantially free of chlorides other than those of titanium and vanadium, and substantially free of non-condensable gases comprising CO, CO2, N2, or mixtures thereof; and (c) separating the impurity present as a solid from the vaporized titanium tetrachloride. The separated vanadium solids may be further processed to recover valuable by products.
Owner:THE CHEMOURS CO TT LLC
Method for preparing vanadium sodium phosphate and vanadium phosphate serving as anode material of sodium ion battery
InactiveCN106410193AReduce process stepsEliminate the step of reducing the pentavalent vanadium source compoundCell electrodesSodium phosphatesPhosphoric acid
The invention provides a method for preparing vanadium sodium phosphate and vanadium phosphate serving as an anode material of a sodium ion battery. The method for preparing the vanadium phosphate comprises the following steps: enabling a trivalent vanadium source compound to react with alkali, thus obtaining vanadium hydroxide, wherein the trivalent vanadium source compound is one of the vanadium sulfate and vanadium chloride or a combination of the vanadium sulfate and the vanadium chloride; enabling the vanadium hydroxide to react with a phosphoric acid, thus obtaining the vanadium phosphate. The method for preparing the vanadium sodium phosphate serving as the anode material of the sodium ion battery comprises the following steps: obtaining the vanadium phosphate prepared by adopting the previous method; mixing the vanadium phosphate with sodium phosphate or sodium carbonate, and sintering, thus obtaining the vanadium sodium phosphate serving as the anode material of the sodium ion battery. The method provided by the invention has the advantages that a pentavalent vanadium source compound having toxicity is replaced by the vanadium sulfate or the vanadium chloride containing trivalent vanadium, so that environment protection is realized; a step for reducing the pentavalent vanadium source compound is omitted, a technological process is shortened, the operation is easy, and industrial production is easy.
Owner:PANZHIHUA IRON & STEEL RES INST OF PANGANG GROUP
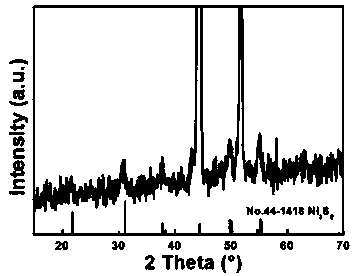
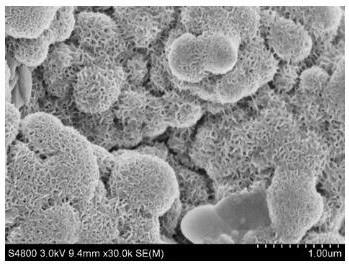
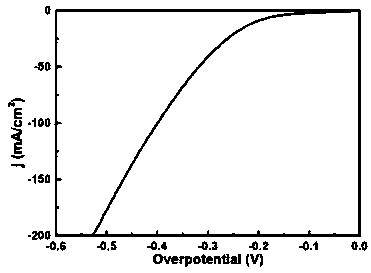
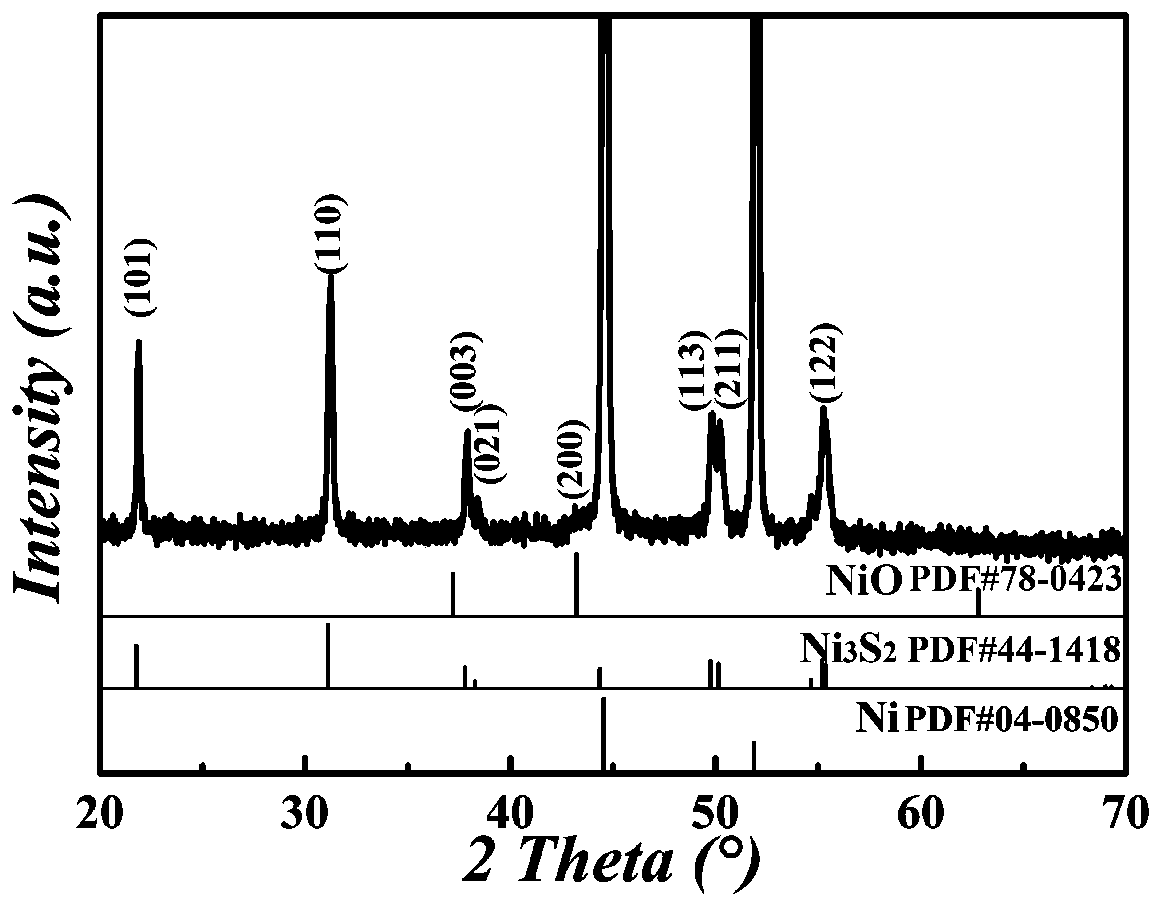
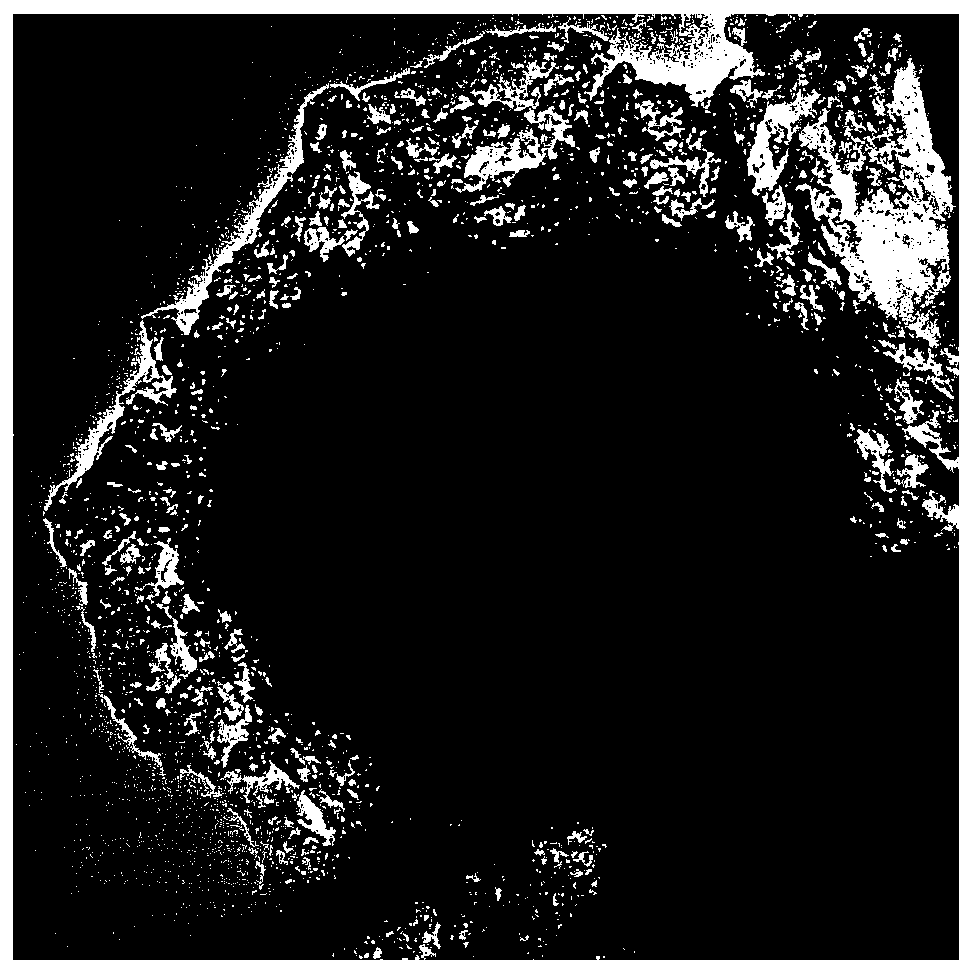
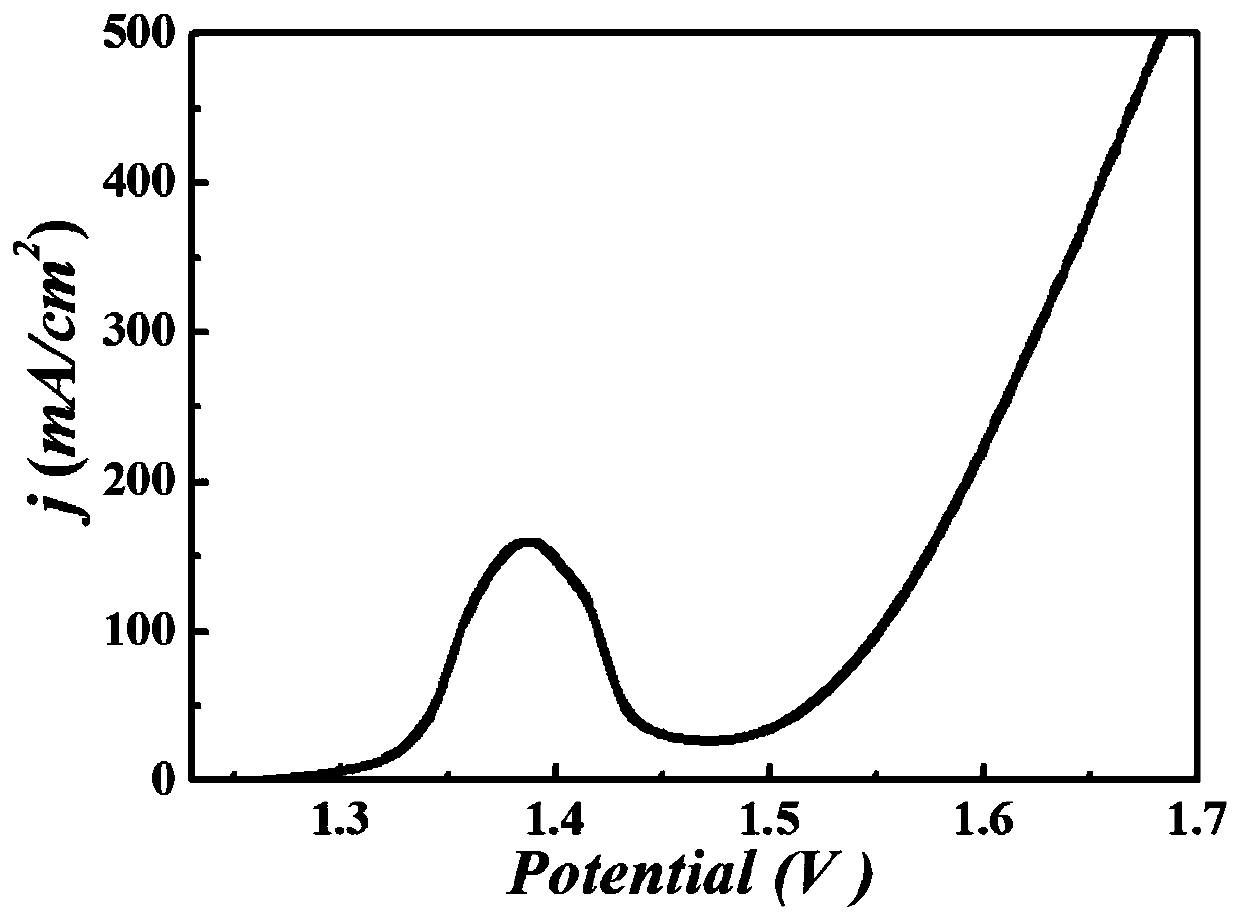
Nanometer forest-shaped V-doped Ni3S2/NF self supporting electrode and preparation method thereof
ActiveCN109267089AHigh catalytic activityThe method steps are simpleElectrode shape/formsAqueous solutionAmmonium fluoride
The invention relates to a nanometer forest-shaped V-doped Ni3S2 / NF self supporting electrode, which comprises a nickel foam substrate and a nanometer forest-shaped V-doped Ni3S2 coating layer growingon the surface of the nickel foam substrate. A preparation method of the self supporting electrode comprises the following steps of soaking clean nickel foam into a precursor solution containing nickel chloride, vanadium chloride, ammonium fluoride and urea; performing first hydrothermal reaction; soaking the reacted nickel foam into a water solution of thioacetamide; performing second hydrothermal reaction at 120 to 160 DEG C for the reaction time of 15 to 24h to obtain the nanometer forest-shaped V-doped Ni3S2 / NF self supporting electrode. The electrode material does not need the use of a bonding agent; the generated electrode material has good OER and HER catalysis activity in a 1M KOH solution.
Owner:SHAANXI UNIV OF SCI & TECH
System and method for producing high-purity vanadium pentoxide through clean chlorination of vanadium resources
ActiveCN109835950AImprove chlorination selectivityEfficient separationEnergy inputVanadium oxidesCatalytic oxidationManganese
The present invention discloses a system and a method for producing high-purity vanadium pentoxide through clean chlorination of vanadium resources. According to the present invention, vanadium resources are subjected to granulation, oxidation, crushing, screening and other pretreatment processes, the pre-treated vanadium resources enter a chlorination furnace and are selectively chlorinated, thevanadium in the vanadium resources is converted into gaseous vanadium oxytrichloride, and most of impurities such as iron, chromium, calcium, phosphorus, manganese, titanium, silicon and the like in the vanadium resources are retained in the chlorinated residue so as to achieve the effective separation of vanadium and other impurities; the gaseous vanadium oxytrichloride is sequentially subjectedto dust removal, leaching, settlement, rectification purification, catalytic oxidation and other processes to prepare the high-purity vanadium pentoxide; the chlorinated residue is sequentially subjected to waste heat recovery, water washing dechlorination, settlement separation, filtering washing and other processes to obtain filter residue and a washing liquid, the filter residue returns to ironsmelting, and the washing liquid is subjected to evaporation crystallization to obtain a chlorine salt; and with the system and the method, the clean comprehensive utilization of vanadium resources is achieved while the sensible heat of the high temperature flue gas of the chlorination furnace and the high temperature chlorination residue is effectively utilized so as to achieve purposes of energy saving and consumption reduction.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Purification of titanium tetrachloride
InactiveUS20060051267A1Reduce lossesZirconium compoundsTitanium tetrachlorideAluminium chlorideTitanium tetrachloride
The present invention is a process for reducing raw material yield losses resulting from the passivation aluminum chloride and vanadium chlorides in the chlorinator discharge in a carbochlorination process for making titanium tetrachloride.
Owner:EI DU PONT DE NEMOURS & CO
Vanadium nitride preparing method
InactiveCN104099634AReduce the temperatureReduce manufacturing costElectrolysis componentsAlkaline earth metalElectrolysis
The invention relates to a vanadium nitride preparing method, belongs to the technical field of non-ferrous metal metallurgy and aims at solving the technical problem and providing the vanadium nitride preparing method. The vanadium nitride preparing method comprises the steps of mixing a vanadium oxide and a carbonaceous reducing agent, using a mixture as an anode, using a carbon steel bar as a cathode, performing electrolysis in an alkali metal / alkaline-earth metal chloride molten salt system containing low-valent vanadium chloride, and leading nitrogen under the cathode, wherein vanadium metal precipitated from the cathode is reacted with the nitrogen to generate vanadium nitride. In the preparing method, the vanadium nitride is obtained through an electrolysis method, the nitridation preparation temperature can be effectively reduced, and the production cost can be reduced. In addition, due to the fact that the refining and protective effect of the electrolysis enables the product quality to be good, the contents of oxygen, carbon and other impurity elements are low. Furthermore, the product particle size can be further adjusted by controlling current density and other parameters and is controllable, and the vanadium nitride preparing method is suitable for preparation of a powdery metallurgical addition agent and has wide application prospect.
Owner:PANZHIHUA IRON & STEEL RES INST OF PANGANG GROUP
Vanadium-based monoatomic catalyst for directly oxidizing benzene to prepare phenol, and preparation method thereof
ActiveCN110124718ALarge specific surface areaEasy to anchorOrganic chemistryOrganic compound preparationBenzeneN dimethylformamide
The invention discloses a vanadium-based monoatomic catalyst for directly oxidizing benzene to prepare phenol, and a preparation method thereof. The catalyst comprises a monoatomic vanadium species derived from a metal-organic framework material NH2-MIL-101 (V), and N-doped amorphous carbon, and the monoatomic vanadium species accounts for 0.1-3% of the weight of the catalyst. The preparation method comprises the following steps: preparing the NH2-MIL-101 (V) from vanadium chloride, 2-aminoterephthalic acid and N,N-dimethylformamide, carrying out high temperature treatment in a N2 atmosphere,and carrying out treatment by using a dilute acid to obtain the vanadium-based monoatomic catalyst V SAs / N-C. The catalyst has an excellent catalytic activity and an excellent reusability when used for directly oxidizing benzene to prepare phenol.
Owner:ZHEJIANG NORMAL UNIVERSITY
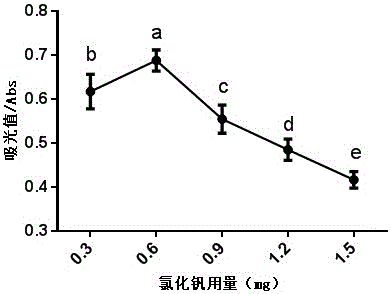
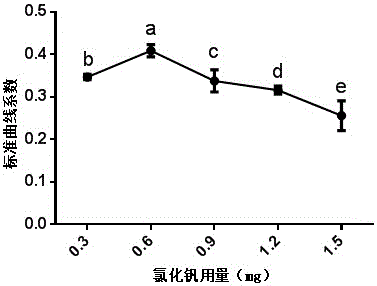
Vanadium trichloride mixed powder and application thereof in quickly measuring nitrate content in food
ActiveCN105954214AReduce consumptionSimple methodColor/spectral properties measurementsEthylic acidWater chlorination
The invention discloses a vanadium trichloride mixed powder and the application thereof in quickly measuring the nitrate content in the food. The vanadium trichloride mixed powder is composed of sodium chloride, sulfamic acid, sulfanilic acid, naphthyl ethylenediamine hydrochloride and vanadium trichloride. When the vanadium trichloride mixed powder is applied to quickly measure the nitrate content in the food, firstly, the food is homogenized and then metered in volume by using the acetic acid solution. After that, the homogenized food is oscillated and filtered to obtain a sample solution. Secondly, the vanadium trichloride mixed powder is added into the sample solution, and the obtained product is shaken uniformly, subjected to water bath and cooled. The light absorption value of the obtained product is measured and then the nitrate content is calculated according to a standard curve. According to the technical scheme of the invention, the method is simple and easy to operate, fast, short in consumed time, sensitive, accurate, small in reagent consumption, and low in cost. Meanwhile, an operator can master the method without being specially trained. Moreover, reagents adopted in the method are not volatilized, free of odors and extremely small in toxicity to human bodies. The mixed power is good in stability. The detection reagent is harmless to the environment and human bodies. Therefore, the vanadium trichloride mixed powder has a wide application prospect in detecting the nitrate content in the rapid detection field of food especially the rapid detection field of fresh vegetables.
Owner:SOUTH CHINA AGRI UNIV
Irregular spherical V-doped Ni3S2/NF oxygen evolution electrocatalyst and preparation method thereof
ActiveCN109277110AEasy to operateLow costPhysical/chemical process catalystsElectrodesOxygen evolutionAqueous solution
Owner:SHAANXI UNIV OF SCI & TECH
Preparation method of vanadium-doped nickel phosphide composite nitrogen-sulfur double-doped reduced graphene oxide electro-catalytic material
ActiveCN111155146ALarge specific surface areaStructural rulesCatalyst activation/preparationElectrodesNickel saltSodium Hypophosphite Monohydrate
The invention discloses a preparation method of a vanadium-doped nickel phosphide composite nitrogen-sulfur double-doped reduced graphene oxide electro-catalytic material. The preparation method comprises the following steps: 1) grinding graphene oxide and L-cysteine; 2) putting a mixture ground in step 1) into a tubular furnace to prepare nitrogen-sulfur double-doped reduced graphene oxide; 3) preparing a nitrogen-sulfur double-doped reduced graphene oxide solution with the concentration of 0.5-1.0 mg / mL; 4) adding urea, NH4F, vanadium chloride and a nickel salt into the nitrogen-sulfur double-doped reduced graphene oxide solution, and stirring until a uniform solution is formed; 5) transferring the solution in step 4) into a reaction kettle to prepare a precursor NiV-LDH / NSG; and (6) putting the precursor NiV-LDH / NSG material and sodium hypophosphite into a tubular furnace together, heating to 300-400 DEG C, and keeping the temperature to obtain the vanadium-doped nickel phosphide composite nitrogen-sulfur double-doped reduced graphene oxide electrocatalytic material NiVP / NSG. The preparation method is low in cost and simple, and the obtained electro-catalytic material has good OER.
Owner:ZHEJIANG UNIV
Ball-flower-like V-doped Ni3S2/NF self-supporting electrode material and preparation method thereof
ActiveCN109280938ALow principle costSimple and fast operationElectrode shape/formsElectricityFlower like
The invention provides a preparation method of a ball-flower-like V-doped Ni3S2 / NF self-supporting electrode material. The preparation method comprises the steps of soaking cleaned foam nickel into apolymeric precursor solution containing nickel chloride hexahydrate, vanadium chloride, ammonium fluoride and urea to conduct primary hydrothermal reaction; soaking the reactive foam nickel into an aqueous solution containing thioacetamide and polyvinyl pyrrolidone to conduct secondary hydrothermal reaction to obtain the ball-flower-like V-doped Ni3S2 / NF self-supporting electrode material. The invention further discloses the ball-flower-like V-doped Ni3S2 / NF self-supporting electrode material. The preparation method provided by the invention is simple in operation, and the reaction period is short. The prepared V-doped Ni3S2 is uniform in morphology and has the self-assembly structure, and the electro-catalytic property of the material is effectively improved.
Owner:SHAANXI UNIV OF SCI & TECH
V-doped Ni3S2/NF electrode material with short rods self-assembled into dendritic shape and preparation method thereof
ActiveCN109371419AHigh catalytic activityMild reaction conditionsElectrode shape/formsMaterials scienceVanadium chloride
The invention provides a V-doped Ni3S2 / NF electrode material with short rods self-assembled into a dendritic shape. The V-doped Ni3S2 / NF electrode material comprises a nickel foam base and V-doped Ni3S2 with the nano-short rods self-assembled into the dendritic shape, and the V-doped Ni3S2 is grown on the nickel foam base. The preparation method of the material comprises the following steps that the cleaned nickel foam is soaked in a precursor solution containing nickel chloride, vanadium chloride, ammonium fluoride and urea, and first-time hydrothermal reaction is performed; the reacted nickel foam is soaked in an ethanol and water mixed solution containing thioacetamide, second-time hydrothermal reaction is performed, and the V-doped Ni3S2 / NF electrode material with the short rods self-assembled into the dendritic shape is obtained. The method has the advantages that the reaction condition is mild, the morphology of the catalyst is regulated and controlled through doping of metal ions, the generated product has a three-dimensional stable structure, and agglomeration is not prone to occurrence in the electrocatalysis process.
Owner:SHAANXI UNIV OF SCI & TECH
Hill-shaped in-situ nickel-vanadium double-metal hydroxide catalyst as well as preparation method and application thereof
ActiveCN110699702AEasy to makeSynthesis temperature is lowElectrode shape/formsMetal/metal-oxides/metal-hydroxide catalystsPtru catalystAlcohol
The invention discloses a hill-shaped in-situ nickel-vanadium double-metal hydroxide catalyst as well as a preparation method and application thereof. The preparation method comprises the following steps: step 1, pretreating foamed nickel; 2, adding vanadium chloride and urea into a mixed solvent of alcohol and Nitrogen-methyl pyrrolidone at the same time; 3, soaking the treated foamed nickel in asolution A, and carrying out solvothermal reaction for 23-25 hours at 115-125 DEG C; and 4, after the reaction is finished, naturally cooling a reaction kettle to room temperature, alternately cleaning with water and alcohol, collecting and drying to obtain the hill-shaped in-situ nickel-vanadium double-metal hydroxide catalyst. The preparation method has the characteristics of simple preparationprocess, low synthesis temperature, no need of large-scale equipment and harsh conditions and the like by adopting the solvothermal method, and the prepared hill-shaped in-situ nickel-vanadium double-metal hydroxide catalyst has high activity and high stability, and has good total water splitting performance under alkaline and neutral conditions.
Owner:SHAANXI UNIV OF SCI & TECH
Preparation method of V-doped NiO coated V-doped Ni3S2 core-shell structure
ActiveCN110615488AStrong structural stabilityFacilitated releaseHydrogen productionHydrogen/synthetic gas productionHydrogenRoom temperature
The invention discloses a preparation method of a V-doped NiO coated V-doped Ni3S2 core-shell structure. The preparation method comprises the steps of: vanadium chloride and urea into ultrapure waterto obtain a solution A; placing the solution A and foamed nickel into a reactor liner, sealing the reactor liner, putting the reactor liner into a homogeneous reaction instrument, carrying out hydrothermal reaction, and cleaning and drying the reaction product to obtain NiV-LDH / NF growing on the foamed nickel in situ; adding a thioacetamide solution NiV-LDH / NF into a hydrothermal kettle, carryingout hydrothermal reaction, and cooling the reaction product to room temperature; and placing the reaction kettle at room temperature for 20-24 hours, taking out the foamed nickel, and cleaning and drying the product to obtain the V-doped Ni3S2 core-shell structure electro-catalytic material coated with V-doped NiO. Foamed nickel is used as a nickel source, and vanadium chloride and urea are used as a vanadium source and an alkali source respectively, so that a NiV-LDH / NF precursor growing on the surface of the foamed nickel in situ is obtained, wherein release of nickel ions in the foamed nickel is accelerated in the presence of vanadium ions; meanwhile, the morphology of NiV-LDH is regulated and controlled, and the obtained in-situ NiV-LDH / NF is extremely high in structural stability. Theprepared composite material with the final core-shell structure has excellent electro-catalytic oxygen evolution and hydrogen evolution performance and stability and high reaction kinetics.
Owner:SHAANXI UNIV OF SCI & TECH
Preparation method of vanadium doped germanate nanowire
InactiveCN103754928AReduced band gapSmall sizeMaterial nanotechnologyGermanium compoundsNanowireVanadium doping
The invention discloses a preparation method of a vanadium doped germanate nanowire and belongs to the technical field of functional materials. The method comprises the following steps: evenly mixing germanium oxide, an acetic acid salt, a vanadium compound with water by taking sodium vanadate, ammonium metavanadate, sodium metavanadate, potassium metavanadate, vanadium oxide or vanadium chloride as a doping source, the germanium oxide and the acetic acid salt as raw materials and the water as a solvent; putting in a reaction container and sealing; and performing heat preservation for 0.5 to 24 hours at 120 DEG C to 180 DEG C, thus obtaining the floccus vanadium doped germanate nanowire. The vanadium doped germanate nanowire has the good visible light photocatalysis property, and has a good application prospect in the fields of industrial wastewater treatment, environment-friendly self-cleaning coatings and self-cleaning glass.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
Method for preparing electronic grade titania ultrafine powder through gas phase oxidation process
ActiveCN107010661AHigh dielectric constantImprove conductivityMaterial nanotechnologyTitanium dioxideDielectricTitanium chloride
The invention relates to a method for preparing electronic grade titania ultrafine powder through a gas phase oxidation process. The method comprises the following steps: reacting titanium chloride vapor, vanadium chloride vapor and oxygen under high-temperature conditions at the temperature of 1500 DEG C or higher so as to obtain a mixture of titania particles containing vanadium oxide and chlorine; performing gas-solid separation on the titania particles and the chlorine, thereby obtaining the electronic grade titania ultrafine powder. The method disclosed by the invention is different from the conventional technology and is short in process procedures, low in production cost and less in environmental pollution, and the produced product is low in impurity content, high in rutile content, high in granularity consistency and high in degree of sphericity. Since a certain ratio of vanadium oxide is introduced into the gas phase oxidation reaction process, the dielectric constant, electrical conductivity and other electrical properties of the titania are greatly improved, the stability of an electronic device is improved, and the operating performance and stability of the titania ultrafine powder in the fields of thermal / pressure sensitive resistors, semiconductor capacitors, battery materials and the like are enhanced.
Owner:SANXIANG ADVANCED MATERIALS
Preparation method of hydrotalcite-based CoNiV composite oxide catalyst and escape ammonia removal application
PendingCN114558580AEnables efficient assembly in situEasy to manufactureHeterogenous catalyst chemical elementsDispersed particle separationHydration reactionPtru catalyst
The invention discloses a preparation method of a hydrotalcite-based CoNiV composite oxide catalyst and an escape ammonia removal application, and belongs to the field of catalyst preparation and application. The preparation method comprises the following steps: by taking cobalt chloride hexahydrate, vanadium chloride and nickel chloride hexahydrate as raw materials, urea as a precipitator and deionized water as a solvent and a detergent, preparing a solution, uniformly stirring, carrying out hydrothermal treatment, carrying out suction filtration, washing and drying to prepare a cobalt-nickel-vanadium hydrotalcite precursor; and roasting the cobalt-nickel-vanadium hydrotalcite precursor at a certain temperature to obtain the catalyst for removing escaping ammonia from the CoNiV composite oxide. The CoNiV composite oxide catalyst prepared by the method disclosed by the invention is applied to low-temperature selective catalytic oxidation escape ammonia (NH3-SCO) reaction and shows good catalytic activity (NH3 conversion rate at 150-360 DEG C reaches 90% or above) and high N2 selectivity.
Owner:JINZHONG UNIV +1
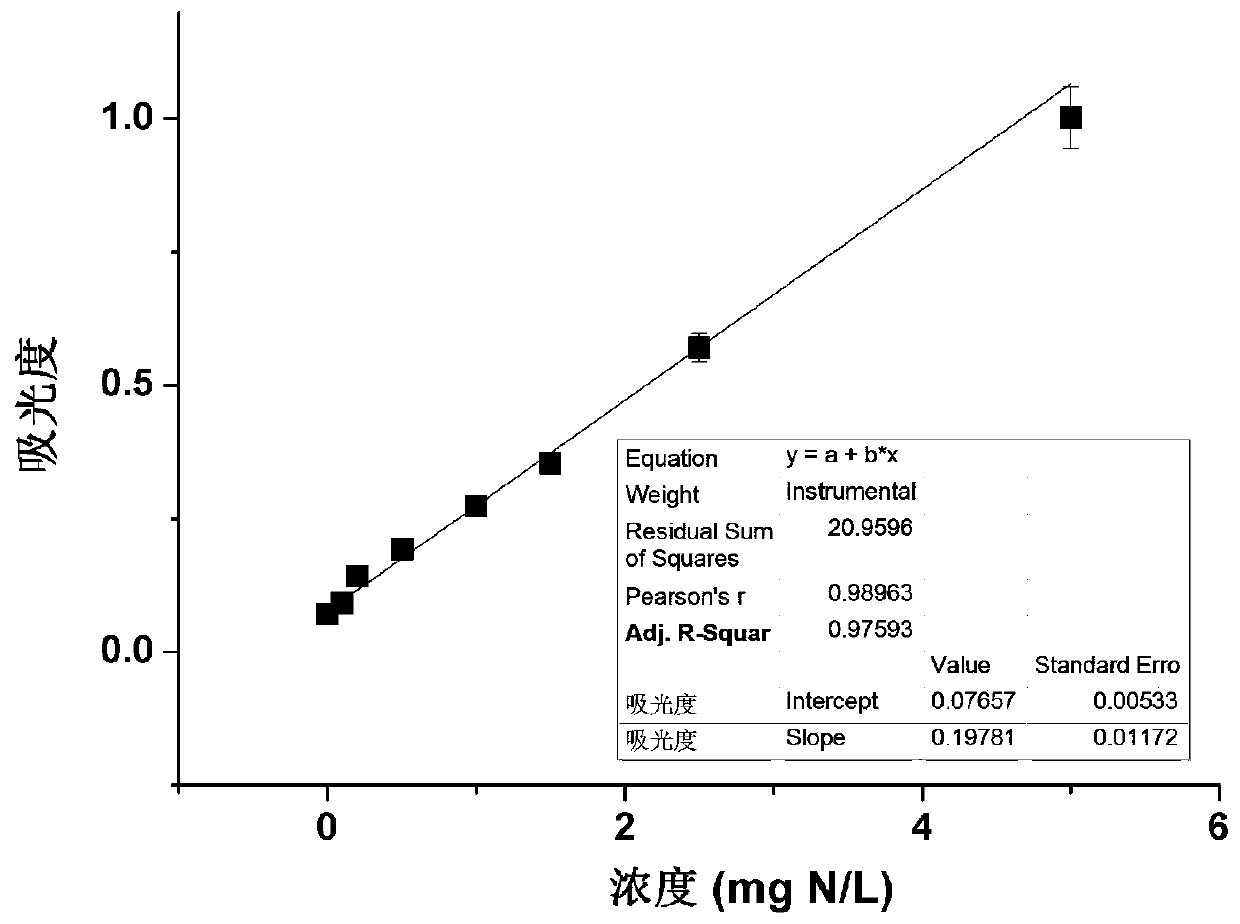
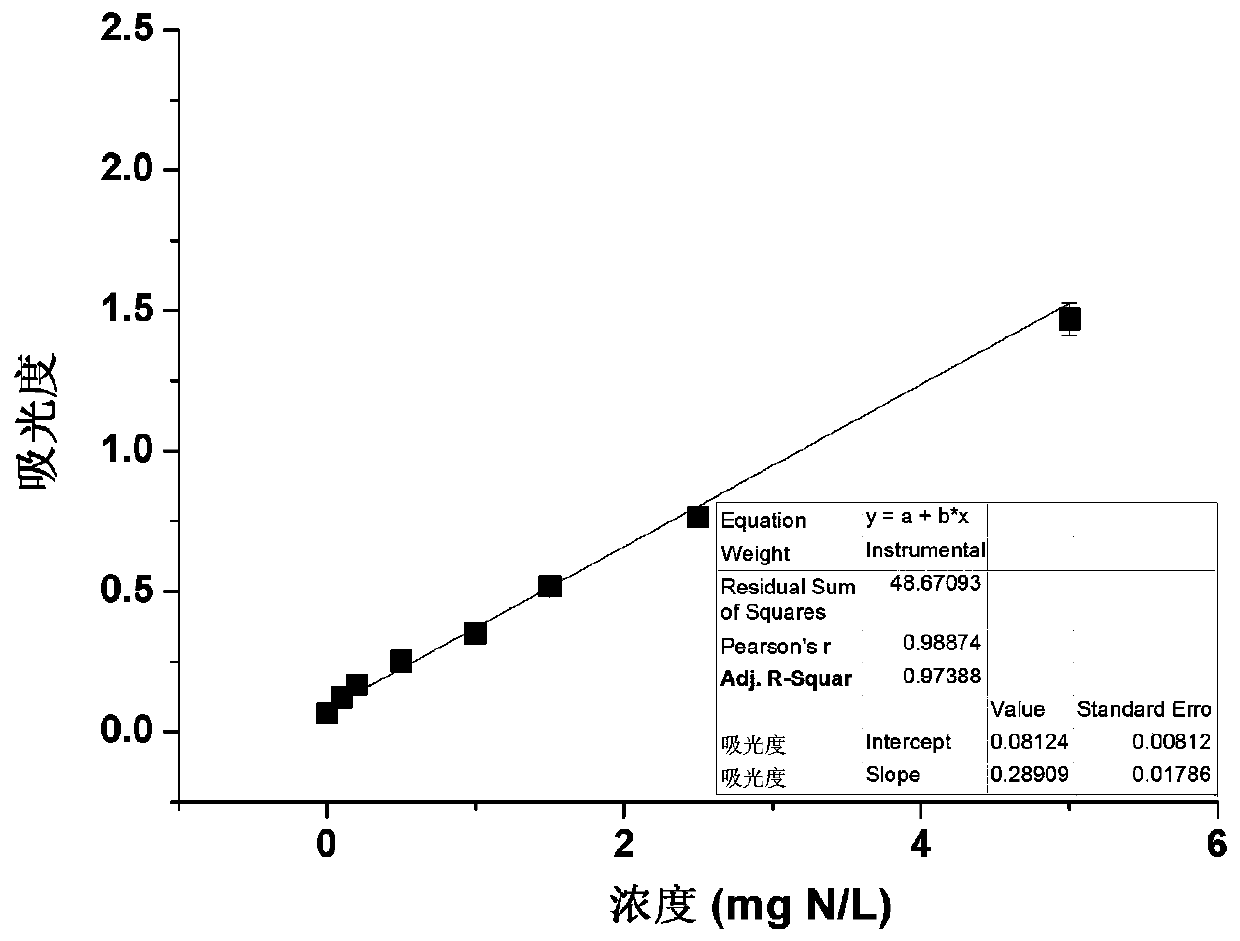
Determination method for ammonium nitrogen, nitric nitrogen, nitrite nitrogen and total dissolved nitrogen in samples
InactiveCN110346361AHigh precisionImprove accuracyMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsETHYLENEDIAMINE DIHYDROCHLORIDESalicylic acid
The invention belongs to the technical field of nitrogen content determination, and provides a determination method for ammonium nitrogen, nitric nitrogen, nitrite nitrogen and total dissolved nitrogen in samples. The method provided by the invention has the advantages that during determination of the ammonium nitrogen, a new oxidant (sodium hypochlorite and sodium chlorinated isocyanurate) and color developer (salicylic acid) system is adopted, the ammonium nitrogen is oxidized into monochloramine under the action of an oxidant, indophenol is obtained from the obtained monochloramine and a color developer, and the ultraviolet absorbance at a wavelength of 660nm is determined for measurement; a new first Griess reagent (N-(1-naphthyl)ethylenediamine dihydrochloride) and second Griess reagent (sulfanilamide and vanadium chloride) system is adopted for measurement of the nitric nitrogen, the nitrite nitrogen and the total dissolved nitrogen, so that low detection limits and high sensitivity are achieved; and the color development reaction is stable in speed and uniform, so as to be carried out on a 96-well plate.
Owner:SHENYANG INST OF APPLIED ECOLOGY - CHINESE ACAD OF SCI
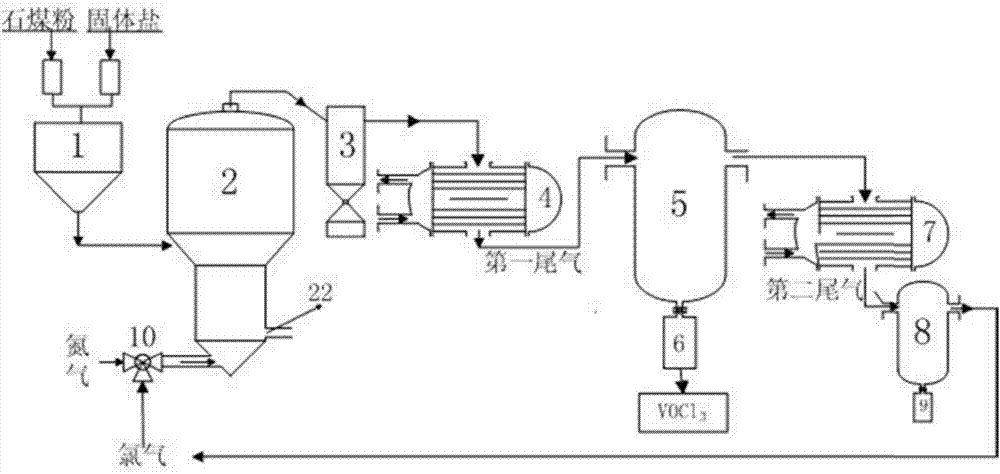
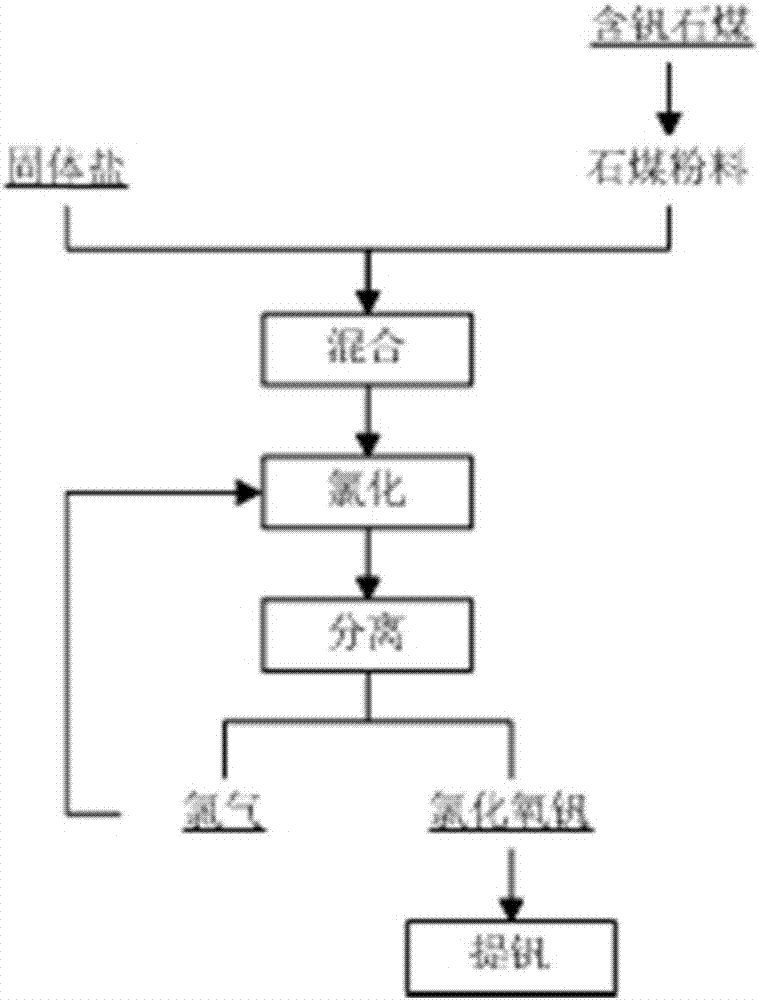
System and method for chlorinating vanadium-containing stone coals
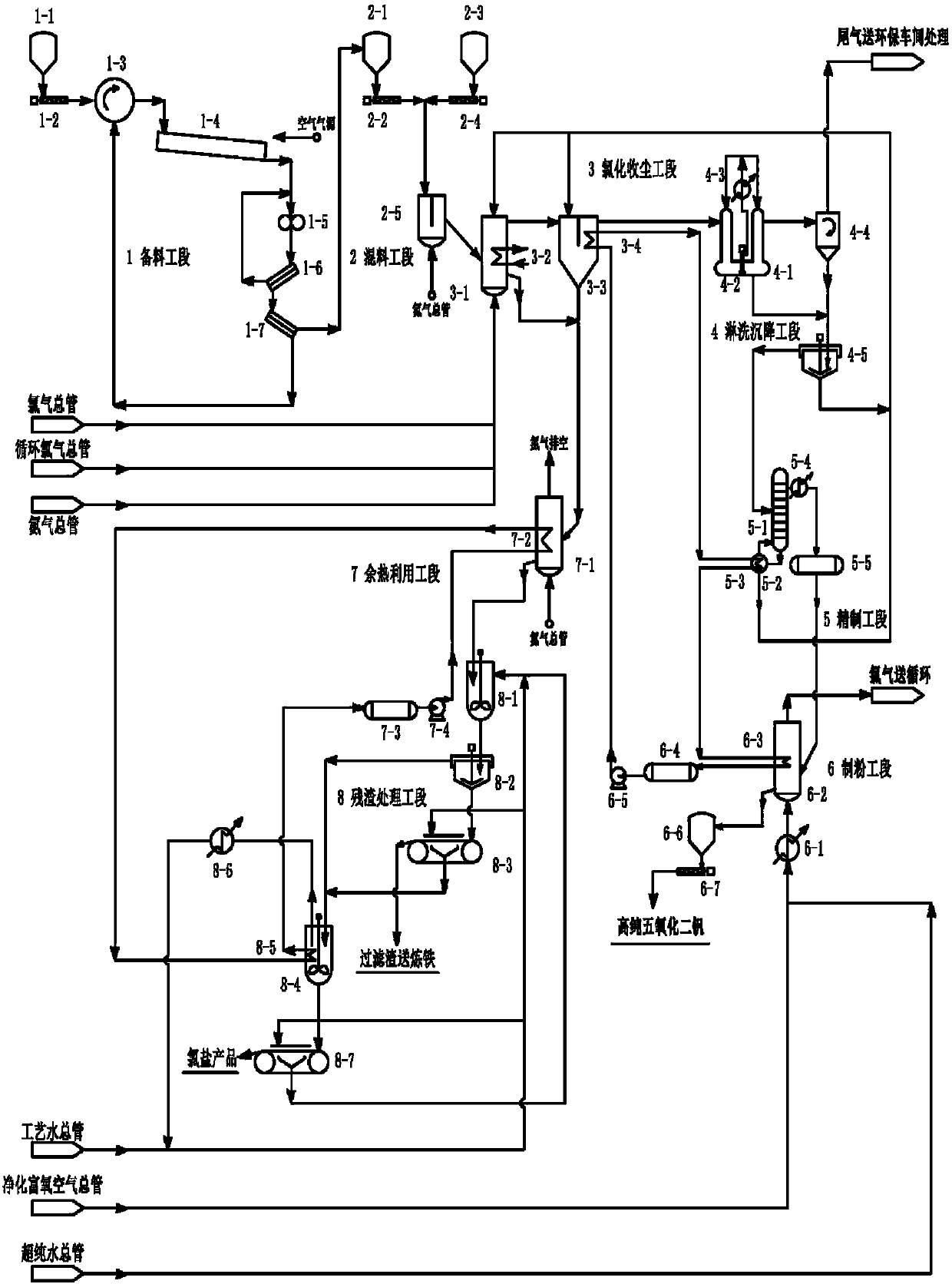
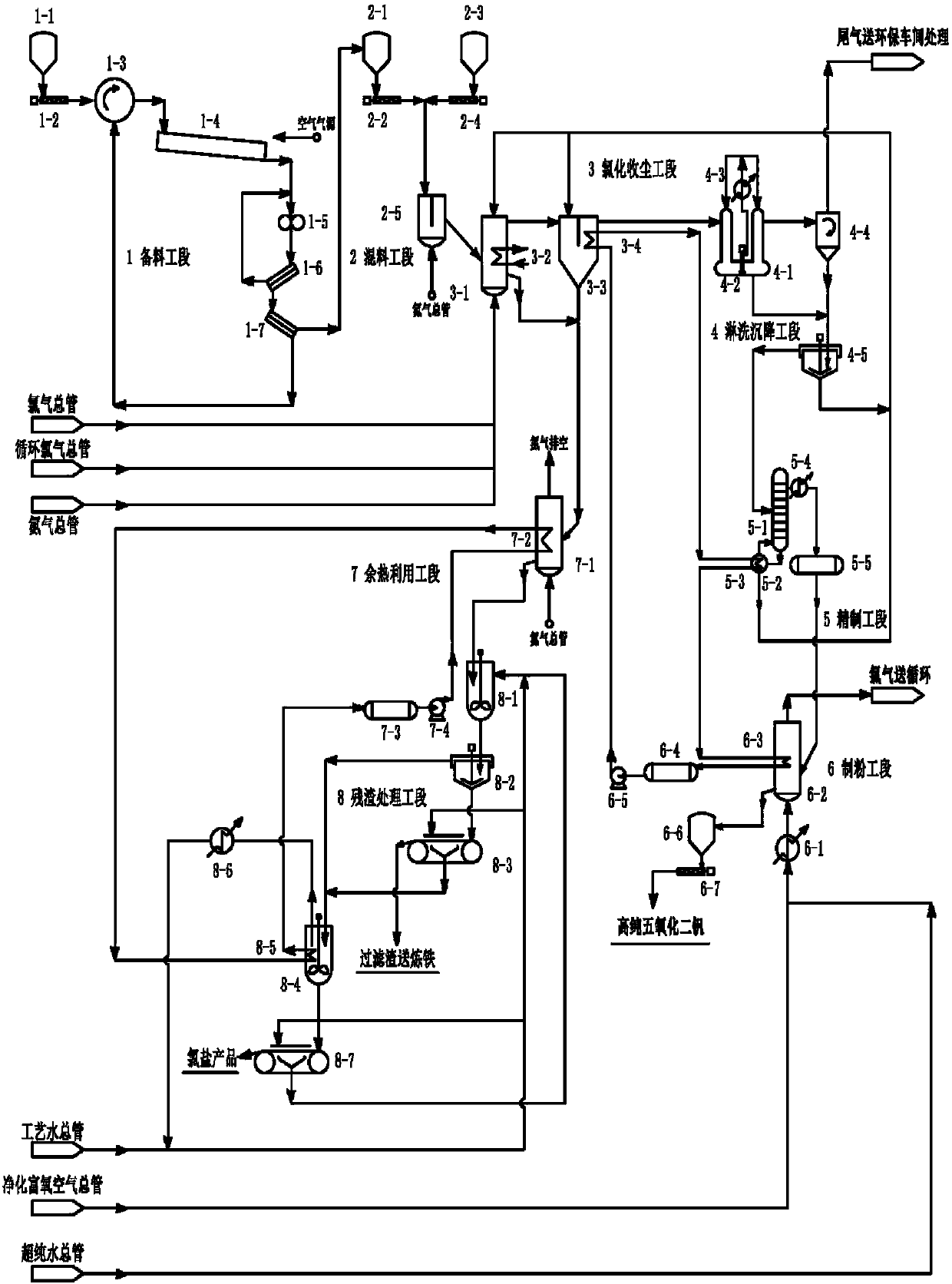
The invention relates to a system used for chlorination of vanadium-containing stone coal. The system includes: a grinding device, a mixing bin, a chlorination system, a dust collector, and a condensation system. The invention also relates to a method for chlorinating vanadium-containing stone coal. Aiming at the characteristics of high carbon, low vanadium and high silicon in stone coal, the present invention uses chlorine gas to add carbochlorination, and adopts condensation means to collect products and remove impurities. At the same time, two elements of vanadium and silicon are recovered, and the main product obtained is vanadium. The content of chloride and vanadium chloride reaches more than 90%, which can significantly reduce the energy consumption of the subsequent treatment steps and improve the economy. Using the chlorination method to treat vanadium-containing stone coal does not produce waste water, the chlorination rate of vanadium is high, and the tail gas can be recycled.
Owner:JIANGSU PROVINCE METALLURGICAL DESIGN INST
Apparatus and method for treating titanium tetrachloride slurry containing vanadium
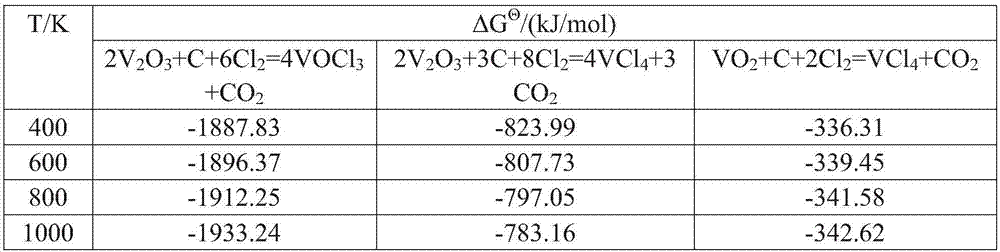
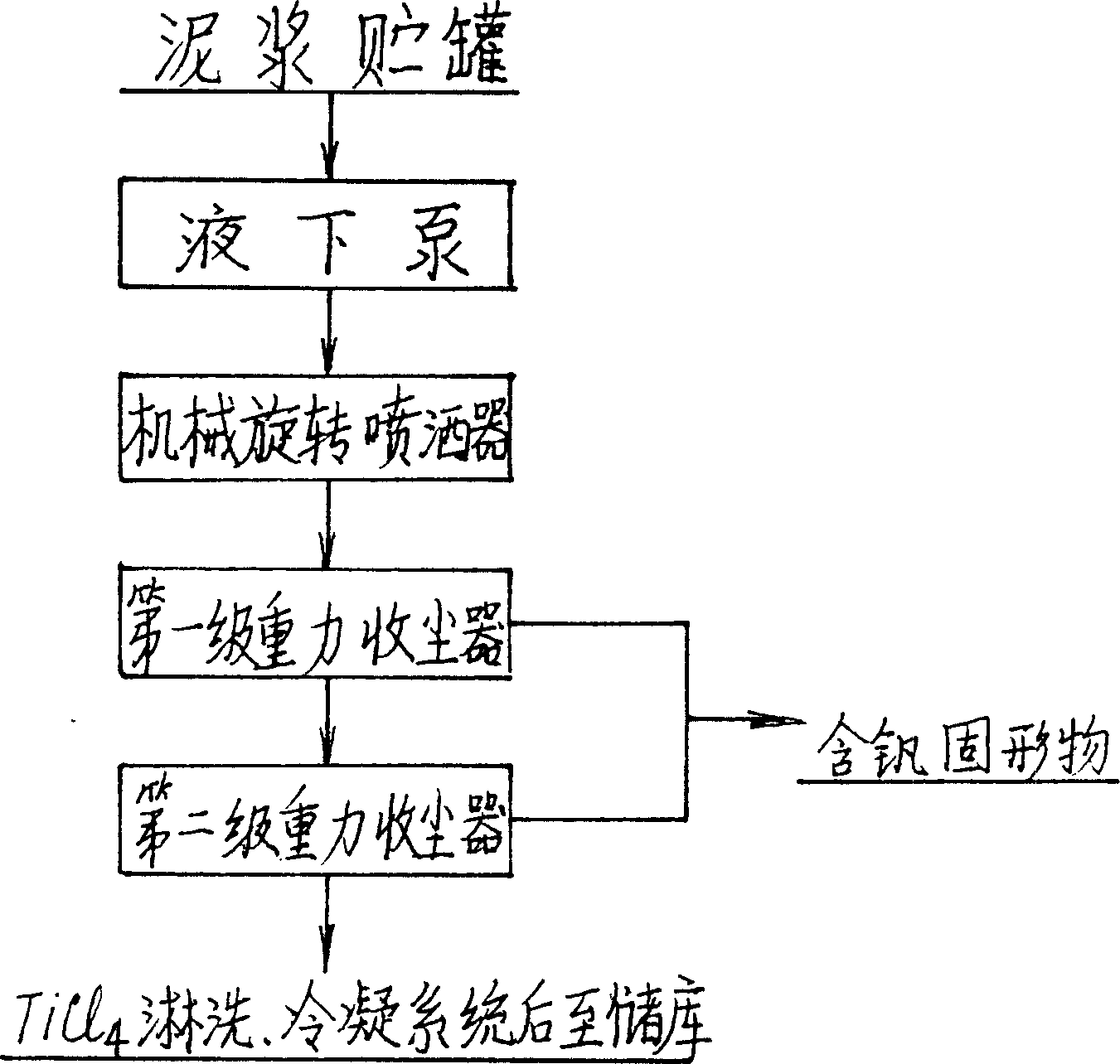
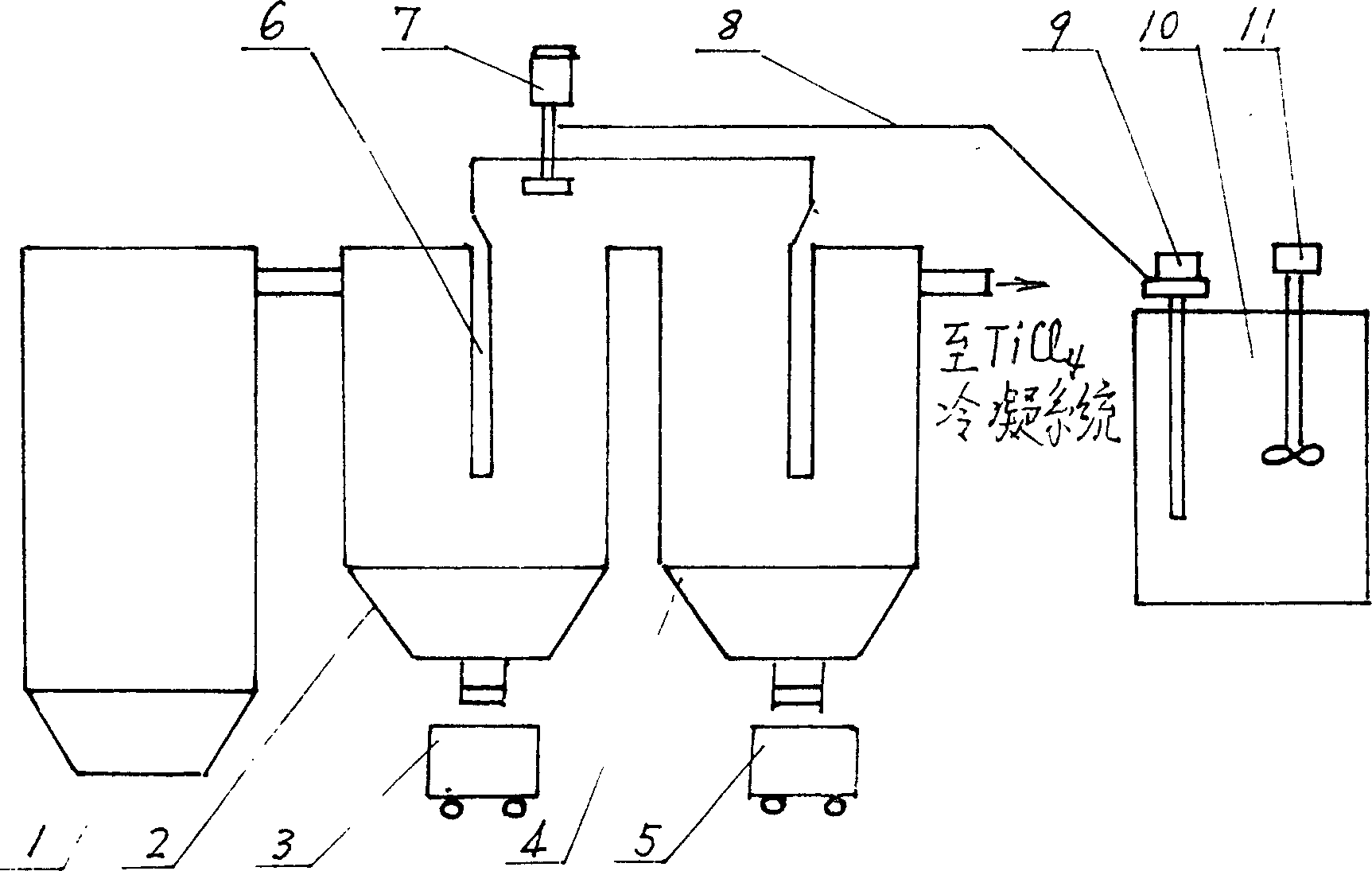
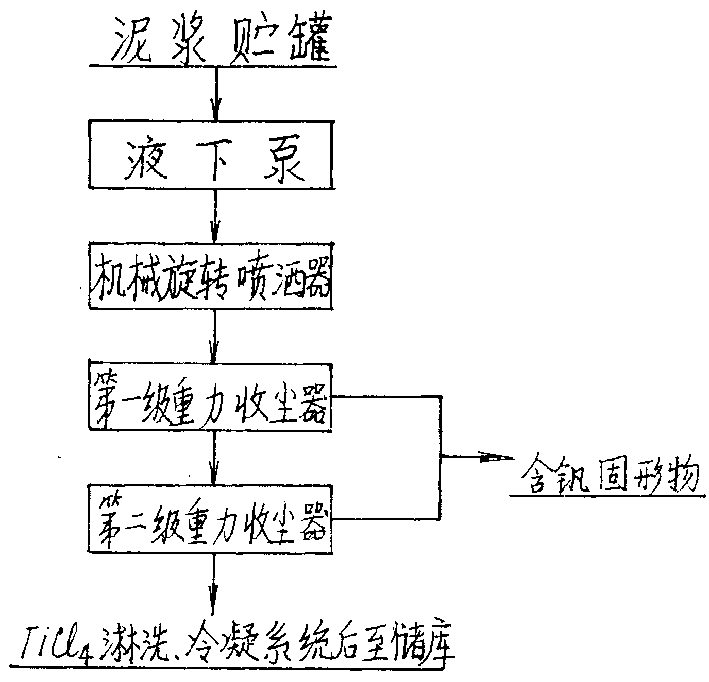
InactiveCN1199863CSave energyLow running costSludge treatmentTitanium dioxideThermal energyBottom ash
A titanium tetrachloride vanadium-containing mud treatment technology and equipment, it uses the device and heat energy of the original chlorination system to treat titanium tetrachloride and vanadium-containing mud in the vanadium-containing mud discharged from the crude titanium tetrachloride mineral oil. Techniques for the separation of vanadium solids. Its feature is to use the submerged pump in the titanium tetrachloride vanadium-containing mud storage tank to send the mud to the mechanical rotary sprayer on the top of the first-stage gravity dust collector of the chlorination system, and then spray the mud into the dust collector and The high-temperature mixed gas discharged from the chlorination furnace is counter-currently exchanged for heat, and the titanium tetrachloride droplets in the mud are gasified, and enter the second-stage dust collector with the mixed gas, and most of the vanadium-containing solids settle under the action of gravity. The ash box at the bottom of the dust collector is sent to the flushing system regularly, and a small amount of unseparated vanadium-containing solids enter the second-stage dust collector with the mixed gas for further separation, and the purified titanium tetrachloride gas enters the original production line. The titanium tetrachloride condensation collection system of the system. The process has the advantages of energy saving, low operation cost, high recovery rate of titanium tetrachloride and no pollution of discharged waste residue.
Owner:中信钛业股份有限公司
Beta-Ni(OH)2 electrode prepared by solvothermal method as well as method and application thereof
ActiveCN112391646ASimple processShort preparation cycleElectrodesElectrolysed waterHexadecyltrimethylammonium bromide
The invention discloses a beta-Ni(OH)2 electrode prepared by a solvothermal method as well as a method and application of the beta-Ni(OH)2 electrode. Ni(OH)2 is vertically arranged on a foamed nickelsheet substrate by adopting a solvothermal method, taking a foamed nickel sheet as a substrate, taking an ethanol solution of vanadium chloride and urea as a vanadium source and taking hexadecyltrimethylammonium bromide as a morphology regulating agent, high-density and large-surface-area catalytic site activity is exposed, the electron transfer rate is increased, the electro-catalytic performanceis improved, the foamed nickel sheet substrate is not only beneficial to electron transmission, nickel comes from an original position, generated Ni(OH)2 is tightly connected with foamed nickel, thestructure is more stable, and the prepared beta-Ni(OH)2 electrode has excellent electro-catalytic performance, and when the beta-Ni(OH)2 electrode is applied to a water electrolysis catalytic reactionunder an alkaline condition, the electro-catalytic hydrogen and oxygen production performance of electrolyzed water is improved.
Owner:SHAANXI UNIV OF SCI & TECH
Vanadium-doped nickel-cobalt double-metal hydroxide electrode material and preparation method thereof
InactiveCN113470985ADoping amount is controllableUniform sizeHybrid capacitor electrodesHybrid/EDL manufactureVanadium dopingCobalt
The invention discloses a vanadium-doped nickel-cobalt double-metal hydroxide electrode material and a preparation method thereof. The preparation method specifically comprises the following steps: adding nickel nitrate hexahydrate, cobalt nitrate hexahydrate and urea into a mixed solvent of methanol and water, and stirring until the solution is clear to obtain a first solution; adding vanadium chloride into the first solution under an ultrasonic or stirring condition to obtain a clarified second solution; and carrying out heat treatment on the second solution, cooling to room temperature, centrifuging to obtain a solid product, and cleaning and drying to obtain the vanadium-doped nickel-cobalt double-metal hydroxide electrode material. According to the invention, the vanadium-doped nickel-cobalt double-metal hydroxide prepared by the method shows a three-dimensional nanoflower-shaped morphology, wherein the nanoflower is composed of a plurality of nanosheets which grow vertically and have the thickness of 10-15 nm; and the preparation method has the characteristics that the preparation process is simple, the size of the obtained nanosheet is uniform, and the doping amount of vanadium is controllable; and the prepared vanadium-doped nickel-cobalt double-metal hydroxide electrode material can be applied to a supercapacitor.
Owner:ZHEJIANG UNIV
Preparation method of cobalt vanadium duplex-metal hydroxide nanosheet as catalyst in electrolysis of water for oxygen evolution
InactiveCN108283929ASynthesis temperature is lowLow costMetal/metal-oxides/metal-hydroxide catalystsElectrodesHexamethylenetetramineOxygen evolution
The invention relates to a preparation method of a cobalt vanadium duplex-metal hydroxide nanosheet as a catalyst in electrolysis of water for oxygen evolution. The preparation method includes the steps that cobalt chloride hexahydrate, vanadium chloride and hexamethylenetetramine are simultaneously added into deionized water to obtain a solution A; the solution A is poured into a reaction still and then sealed, the reaction still is placed in an outer kettle, and then the outer kettle is placed in a homogeneous reactor for a reaction; after the reaction is completed, the reaction still is naturally cooled to room temperature, and a product obtained after the reaction is alternately washed with water and alcohol, collected and dried under vacuum to obtain the CoV-LDH nanosheet. A one-stephydrothermal method is adopted, the synthesis temperature is low, the yield is high, no post-treatment is needed, and the environment is protected. The prepared CoV-LDH is a two-dimensional nanosheetwhich is beneficial to free ion in and out, full contact of an electrolyte and the CoV-LDH nanosheet is facilitated, more active sites can be exposed, and correspondingly the electrochemical performance can be improved. In addition, the chemical composition of the product is uniform, the purity is high, the morphology is uniform, the nanosheet can show excellent electrochemical performance when used as an electrode material in electrolysis of water, and at the current density of 10mA / cm<2>, the overpotential is about 280 mV.
Owner:SHAANXI UNIV OF SCI & TECH
Method for preparing lithium vanadium phosphate (positive electrode material) of lithium ion cell
InactiveCN106328943AProcess environmental protectionShort processCell electrodesSecondary cellsLithium vanadium phosphate batteryLithium hydroxide
The invention provides a method for preparing a lithium vanadium phosphate (positive electrode material) of a lithium ion cell. The method comprises the following steps: carrying out reaction on trivalent vanadium source compound and alkali, thereby acquiring vanadium hydroxide, wherein the trivalent vanadium source compound is vanadium sulphate and / or vanadium chloride; carrying out reaction on vanadium hydroxide and phosphoric acid, thereby acquiring vanadium phosphate; mixing vanadium phosphate with lithium hydroxide or lithium carbonate and sintering, thereby acquiring the lithium vanadium phosphate (positive electrode material) of the lithium ion cell. According to the method, instead of the toxic pentavalent vanadium source compound, trivalent vanadium sulphate or vanadium chloride is utilized to prepare vanadium phosphate, so that the technique is more environmentally friendly. The step of reducing the pentavalent vanadium source compound is avoided in the vanadium phosphate preparation process, so that the technological process is shortened, the operation is simple and the industrial production is easily realized.
Owner:PANZHIHUA IRON & STEEL RES INST OF PANGANG GROUP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com