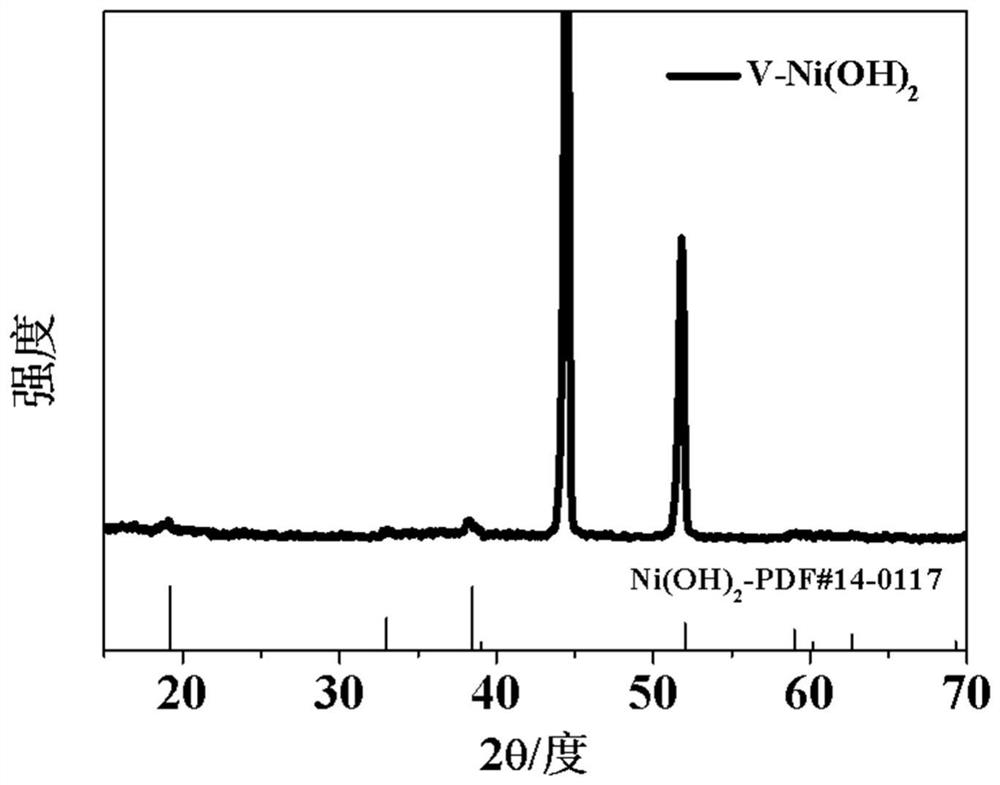
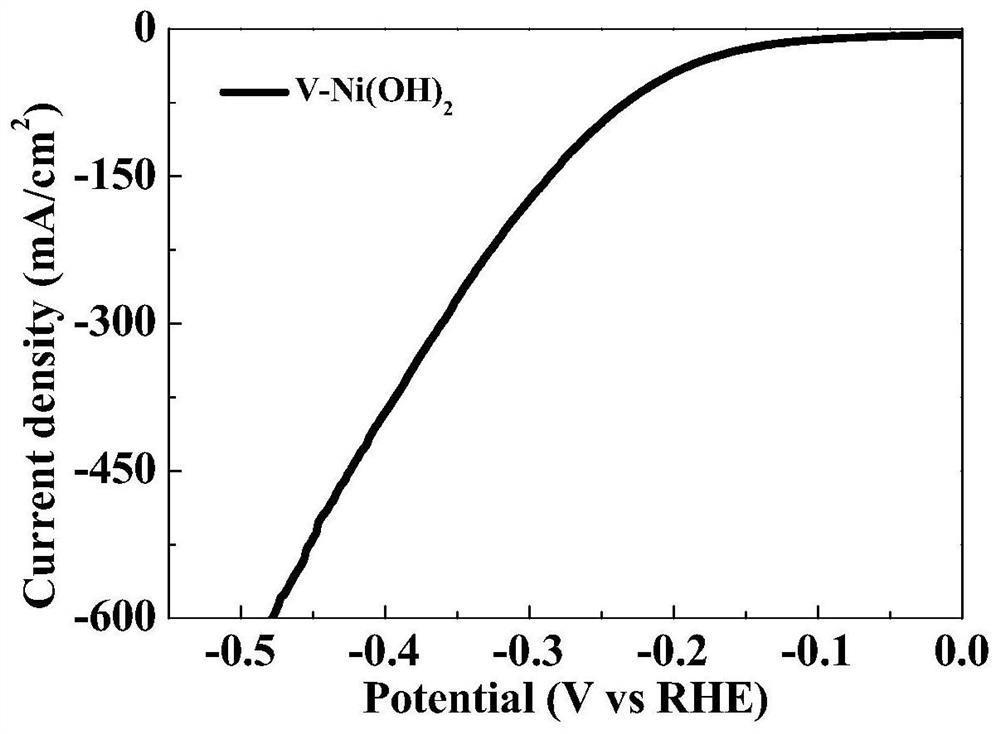
Beta-Ni(OH)2 electrode prepared by solvothermal method as well as method and application thereof
A solvothermal and electrode technology, applied in electrodes, electrolysis process, electrolysis components, etc., to achieve the effect of simple process, short preparation period and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1) Cut the nickel foam into 1.5×4.0cm 2 Rectangular slices were cleaned and dried at room temperature in a vacuum oven;
[0038] 2) The molar ratio is (2~3):1 Measure deionized water and absolute ethanol, prepare 20mL of ethanol solution, weigh 38.753~39.567g of vanadium chloride, 73~77mg of urea, dissolve them in deionized In a solution composed of water and absolute ethanol, stir at room temperature for 30 minutes, then add 210-220 mg of treated foam nickel to obtain a mixed solution;
[0039] 3) Then add 0.345-0.465 g of hexadecyltrimethylammonium bromide morphology control agent to the mixed solution, stir at room temperature for 30 minutes, then place the mixed solution in an oven at 155° C. for 21 hours;
[0040] 4) After the reaction is over, after naturally cooling to room temperature, take out the nickel foam, and rinse it with ultrapure water and absolute ethanol five times;
[0041] 5) Finally, put the rinsed nickel foam into a vacuum oven to dry at room te...
Embodiment 2
[0044] 1) Cut the nickel foam into 1.5×4.0cm 2 Rectangular slices were cleaned and dried in a vacuum box at room temperature;
[0045] 2) Prepare 20 mL of ethanol solution with deionized water and absolute ethanol at a molar ratio of (2-3):1, weigh 38.753-39.567 g of vanadium chloride and 73-77 mg of urea, and dissolve them in the ethanol solution , stirred at room temperature for 30 minutes, and then put in 210-220 mg of treated foam nickel to obtain a mixed solution;
[0046] 3) Then add 0.345-0.465 g of hexadecyltrimethylammonium bromide morphology control agent to the mixed solution, stir at room temperature for 30 minutes, and then place the mixed solution in an oven at 153° C. for 22 hours;
[0047] 4) After the reaction is over, after naturally cooling to room temperature, take out the nickel foam, and rinse it with ultrapure water and absolute ethanol five times;
[0048] 5) Finally, put the rinsed nickel foam into a vacuum oven to dry at room temperature for 8 hours...
Embodiment 3
[0051] 1) Cut the nickel foam into 1.5×4.0cm 2 Rectangular slices were cleaned and dried in a vacuum box at room temperature;
[0052] 2) Prepare 20 mL of ethanol solution with deionized water and absolute ethanol at a molar ratio of (2-3):1, weigh 38.753-39.567 g of vanadium chloride and 73-77 mg of urea, and dissolve them in the ethanol solution , stirred at room temperature for 30 minutes, and then put in 210-220 mg of treated foam nickel to obtain a mixed solution;
[0053] 3) Then add 0.345-0.465 g of hexadecyltrimethylammonium bromide morphology control agent to the mixed solution, stir at room temperature for 30 minutes, then place the mixed solution in an oven at 150° C. for 23 hours;
[0054] 4) After the reaction is over, after naturally cooling to room temperature, take out the nickel foam, and rinse it with ultrapure water and absolute ethanol five times;
[0055] 5) Finally, put the rinsed nickel foam into a vacuum oven to dry at room temperature for 8 hours to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com