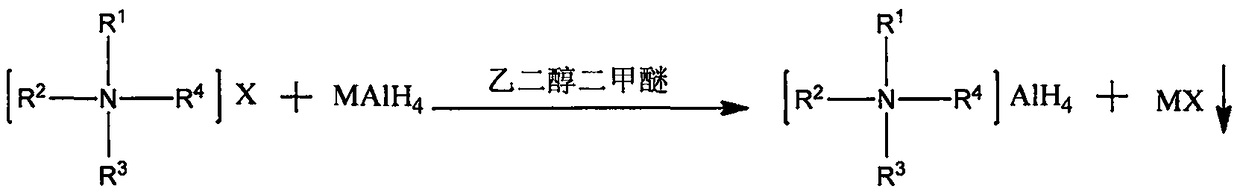
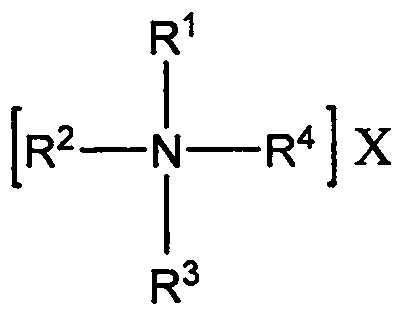
Method for preparing quaternary ammonium aluminum hydride
A technology of aluminum hydride and quaternary ammonium, applied in the field of preparation of quaternary ammonium aluminum hydride, can solve the problems of poor solubility, difficulty in purification, and inability to obtain products effectively, and achieves the effect of avoiding filtration of impurity salts and shortening reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Under nitrogen protection, weigh 1.56g LiAlH 4 (0.0411mol) in a 500mL three-necked flask, add 80mL of anhydrous ethylene glycol dimethyl ether, and install a reflux condenser and a constant pressure dropping funnel; then weigh 15g of tetra-n-octylammonium bromide (0.0274mol) in Add 80mL of anhydrous ethylene glycol dimethyl ether to a 250mL flask with a branch, heat and stir in an oil bath at 50°C to dissolve; then, transfer the tetra-n-octylammonium bromide solution to a constant pressure dropping funnel Add dropwise to the there-necked flask within 40min; after the dropwise addition, continue to heat and reflux for 4h; after the reaction, add 160mL of n-heptane and filter through a sand core funnel to obtain a transparent and clear filtrate; then carry out vacuum distillation on the filtrate, Most of the solvent was removed, and finally vacuum-dried in an oil bath at 55° C. for 2 h to obtain 12.94 g of a waxy solid with a yield of about 94.9%.
Embodiment 2
[0031] Under nitrogen protection, weigh 0.78g LiAlH 4 (0.0206mol) in a 250mL three-neck flask, add 50mL of anhydrous ethylene glycol dimethyl ether, and install a reflux condenser and a constant pressure dropping funnel; then weigh 5.54g of tri-n-octylmethyl ammonium chloride (0.0137 mol) into a 250mL flask with a branched mouth, then add 50mL of anhydrous ethylene glycol dimethyl ether, and heat and stir in an oil bath at 50°C to dissolve; subsequently, transfer the tri-n-octylmethylammonium chloride solution to In the constant pressure dropping funnel, add it dropwise into the three-neck flask within 40 minutes, and the solution begins to form a large amount of white precipitate; after the dropwise addition, continue to heat and reflux for 2 hours; Transparent and clear filtrate; the filtrate was distilled under reduced pressure to remove most of the solvent, and finally dried in vacuum in an oil bath at 55° C. for 2 hours to obtain 5.10 g of a waxy solid with a yield of abo...
Embodiment 3
[0033] Under nitrogen protection, weigh 1.11g NaAlH 4 (0.0206mol) in a 500mL three-necked flask, add 80mL tetrahydrofuran, and install a reflux condenser and a constant pressure dropping funnel; then weigh 10.85g hexadecyldimethylbenzyl ammonium chloride (0.0274mol) in 250mL Add 80 mL of anhydrous ethylene glycol dimethyl ether to a flask with a branched mouth, heat and stir in an oil bath at 50°C to dissolve; then, transfer the cetyl dimethyl benzyl ammonium chloride solution to a constant In the pressure dropping funnel, drop it into the three-necked bottle within 40 minutes, and the solution begins to form a large amount of white precipitate; after the dropwise addition, continue to heat and reflux for 4 hours; The clarified filtrate; the filtrate was distilled under reduced pressure to remove most of the solvent, and finally vacuum-dried in an oil bath at 55° C. for 2 hours to obtain 10.32 g of a waxy solid with a yield of about 96.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com