Anion-cation co-doped zinc oxide conductive powder and preparation method thereof
An anion-cation, conductive powder technology, applied in the direction of zinc oxide/zinc hydroxide, conductive coatings, etc., can solve the problem that the whiteness of light-colored conductive powder doped with zinc oxide is not high enough, it is unfavorable for large-scale industrial production, and the preparation process is complicated. It can improve the stability, reduce the absorption ratio and improve the whiteness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1) Accurately weigh 29.75g Zn(NO 3 ) 2 ·6H 2 O and 0.38g Al (NO 3 ) 3 ·9H 2 O, dissolved in 100ml of deionized water, stirred for 20 minutes to form a homogeneous and stable solution A, weigh 20g of NH 4 HCO 3 Dissolve in 100ml of deionized water, stir for 20 minutes to form a homogeneous and stable solution B, slowly add solution B dropwise to solution A, the drop rate is 10ml / min, and keep stirring, the stirring rate is 600r / min; control the reaction at the same time The temperature of the solution is between 20°C and 40°C, and the pH value of the reaction solution is monitored. When the pH value of the solution is close to neutral, the dropwise addition of solution B is stopped, and the stirring is continued for 20 minutes to obtain precipitation;
[0038] 2) The precipitate obtained in step 1) was dried at 120° C. for 12 hours after being filtered, washed with water 4 times, washed with ethanol 2 times (the volume ratio of water or ethanol used for washing and...
Embodiment 2
[0043] Accurately weigh 29.75g Zn(NO 3 ) 2 ·6H 2 O and 0.46gGa (NO 3 ) 3 ·9H 2 O, dissolved in 100ml of deionized water, stirred for 20 minutes to form a homogeneous and stable solution A, weigh 20g of NH 4 HCO 3Dissolved in 100ml of deionized water, stirred for 20 minutes to form a homogeneous and stable solution B, other methods were the same as in Example 1, except that 0.4g ZnF was used before calcination 2 Replace AlF 3 , and then mixed with the dried co-precipitated product and ball-milled for 2 hours.
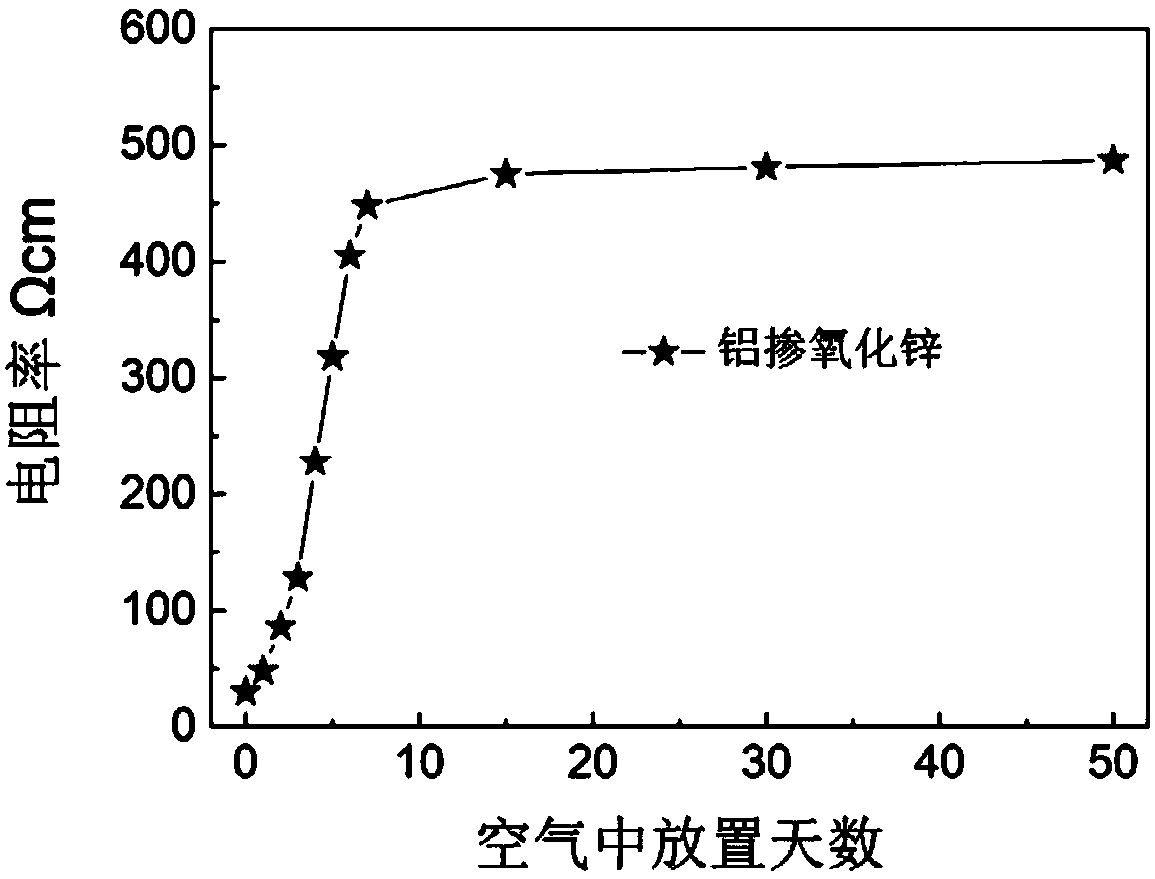
[0044] The whiteness of the obtained powder is 88, the volume resistivity is 10Ωcm, the volume resistivity is 55Ωcm after being placed in the natural environment for 50 days, and the volume resistivity is 132Ωcm when the air environment is heated to 500°C.
[0045] Comparative experiment without fluorine doping: other methods are the same as above, except that ZnF is not doped during the experiment 2 powder.
[0046] The whiteness of the obtained powder is 71, ...
Embodiment 3
[0048] Accurately weigh 29.75g Zn(NO 3 ) 2 ·6H 2 O and 0.59g In (NO 3 ) 3 ·9H 2 O was dissolved in 100ml of deionized water, stirred for 20 minutes to form a homogeneous and stable solution A, weigh 20g of NH 4 HCO 3 Dissolved in 100ml of deionized water and stirred for 20 minutes to form a homogeneous and stable solution B. Other methods are the same as in Example 1, except that 0.4g of ZnF 2 It was mixed with the dried coprecipitated product and ball milled for 2 hours.
[0049] The whiteness of the obtained powder was 81, the volume resistivity was 31Ωcm, the volume resistivity was 98Ωcm after being placed in the natural environment for 50 days, and the volume resistivity was 180Ωcm when the air environment was heated to 500°C.
[0050] Comparative experiment 3 without fluorine doping: other methods are the same as above, except that ZnF is not doped during the experiment 2 powder.
[0051] The whiteness of the obtained powder is 65, the volume resistivity is 136Ωc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| whiteness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com