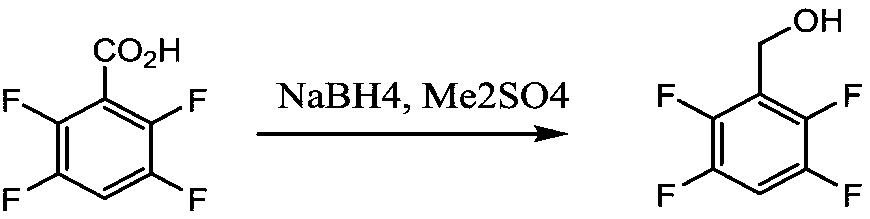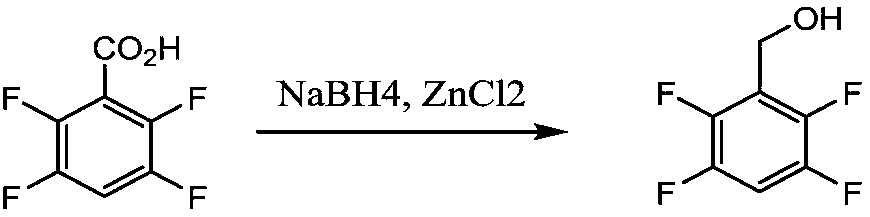Preparation method of tetrafluorobenzene methanol
A technology of tetrafluorobenzyl alcohol and tetrafluorobenzoyl chloride, which is applied in the field of preparation of tetrafluorobenzyl alcohol and benzyl alcohol, can solve the problems of high raw material cost, achieve high activity, reduce the probability of esterification side reactions, and promote reduction The effect of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Synthesis of 2,3,5,6-tetrafluorobenzyl alcohol
[0043] Add 3.8g (0.1mol) of NaBH4 and 12g of ethylene glycol dimethyl ether into a 500mL four-necked flask, stir and heat to 78-80°C under nitrogen protection, and reflux for 3 hours.
[0044] After the activation reaction, cool down to 20-30°C, add 300g of water, stir to dissolve, cool down to 5°C, add dropwise 54.6g of 2,3,5,6-tetrafluorobenzoyl chloride obtained in Comparative Example 1 under nitrogen protection. The reaction is strongly exothermic, and gas and solids are generated. The reaction temperature is controlled at 5-15°C, and the dropwise addition takes about 3 hours. After the dropwise addition, the mixture was kept at this temperature for 1 hour.
[0045] Add 100g of dichloromethane and stir for 10 minutes, filter out insoluble inorganic salts, rinse the filter cake with 50g of dichloromethane, combine the filtrates, separate the organic layer, distill and recover the dichloromethane to obtain wh...
Embodiment 2
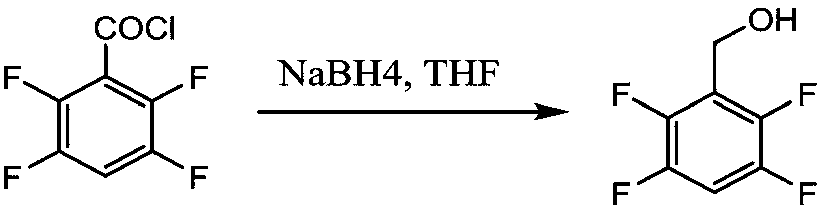
[0047] Example 2 Synthesis of 2,3,4,5-tetrafluorobenzyl alcohol
[0048] Add 50g (0.258mol) of 2,3,4,5-tetrafluorobenzoic acid to 150mL of thionyl chloride, drop 3 drops of DMF, and heat up to 70°C for reflux reaction. When the solution becomes clear and no gas is released Time to view the end of the reaction. Atmospheric distillation was carried out to recover thionyl chloride, followed by vacuum distillation to obtain 54.1 g of a colorless oily product, 2,3,4,5-tetrafluorobenzoyl chloride, with a yield of 99%.
[0049] Add 4.16g (0.11mol) of NaBH4 and 10g of ethylene glycol dimethyl ether into a 500mL four-neck flask, stir and heat to 78-80°C under nitrogen protection, and reflux for 3 hours.
[0050]After the activation reaction, cool down to 20-30°C, add 300g of water, stir to dissolve, cool down to 5°C, and add 54.1g of 2,3,4,5-tetrafluorobenzoyl chloride dropwise under nitrogen protection. The reaction is strongly exothermic, and gas and solids are generated. The react...
Embodiment 3
[0052] Example 3 Synthesis of 2,4,5,6-tetrafluorobenzyl alcohol
[0053] Add 50g (0.258mol) of 2,4,5,6-tetrafluorobenzoic acid to 150mL of thionyl chloride, drop 3 drops of DMF, and heat up to 70°C for reflux reaction. When the solution becomes clear and no gas is released Time to view the end of the reaction. Atmospheric pressure distillation was used to recover thionyl chloride, followed by vacuum distillation to obtain 2,4,5,6-tetrafluorobenzoyl chloride 54.1 as a colorless oily product with a yield of 99%.
[0054] Add 4.44g (0.12mol) of NaBH4 and 13g of ethylene glycol dimethyl ether into a 500mL four-neck flask, stir and heat to 78-80°C under nitrogen protection, and reflux for 3 hours.
[0055] After the activation reaction, cool down to 20-30°C, add 300g of water, stir to dissolve, cool down to 5°C, and add 54.1g of 2,4,5,6-tetrafluorobenzoyl chloride dropwise under nitrogen protection. The reaction is strongly exothermic, and gas and solids are generated. The reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com