Synthetic method of 2-trifluoromethylindenemethylindole derivative
A technology of trifluoromethylindenylmethyleneindole and synthesis method, which is applied in the field of synthesis of 2-trifluoromethylindenylmethyleneindole derivatives to protect the health of environmental operators and achieve high yield efficiency, and the effect of saving synthesis costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
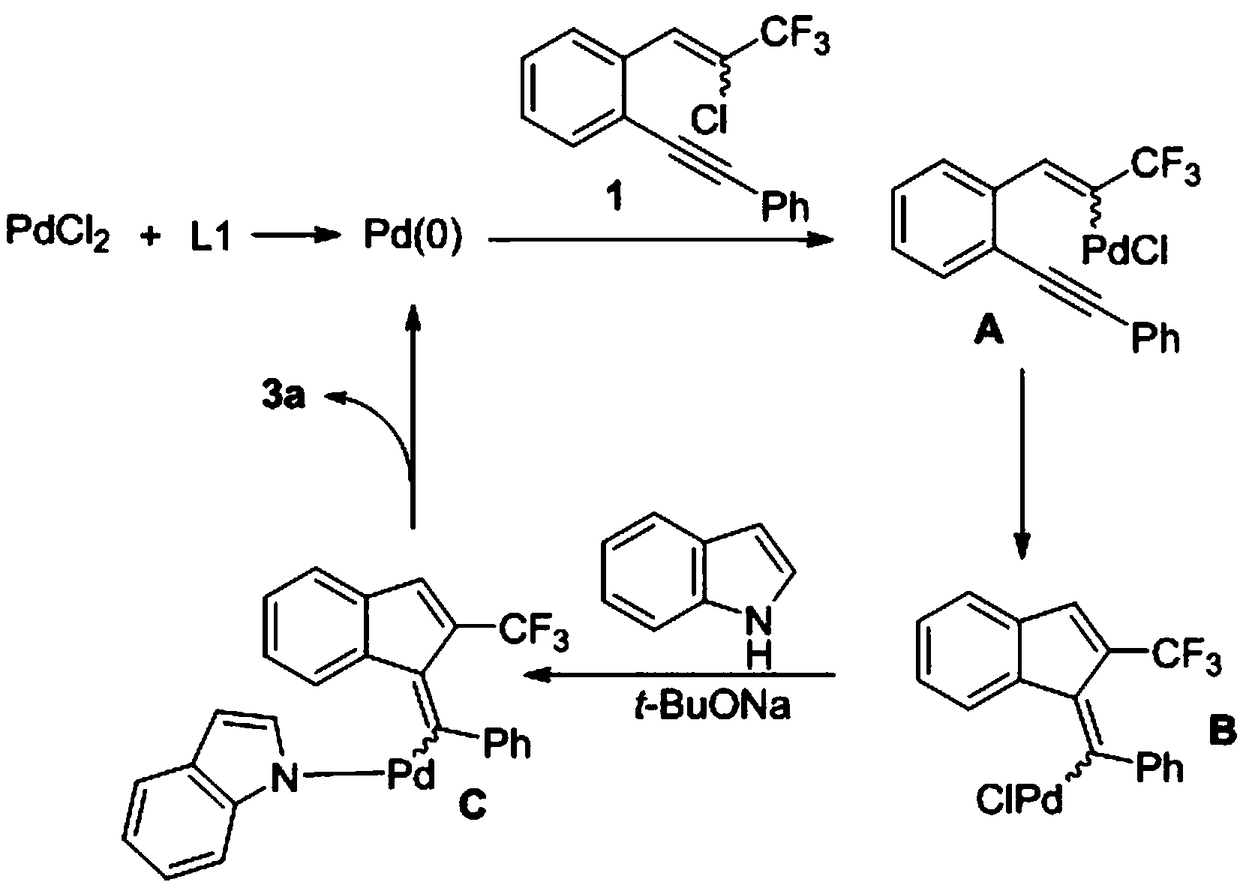
Method used
Image
Examples
specific Embodiment 1
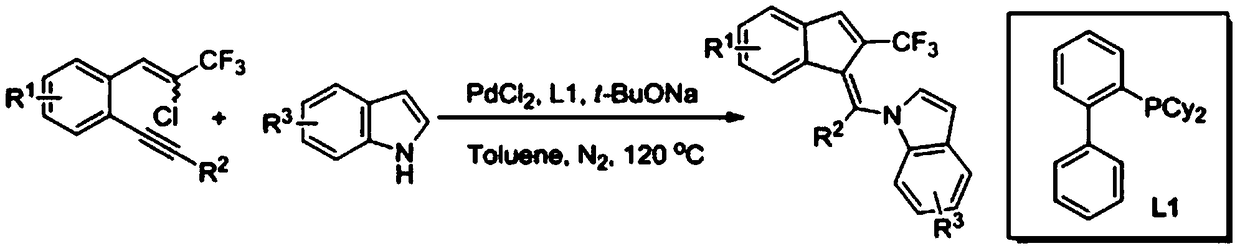
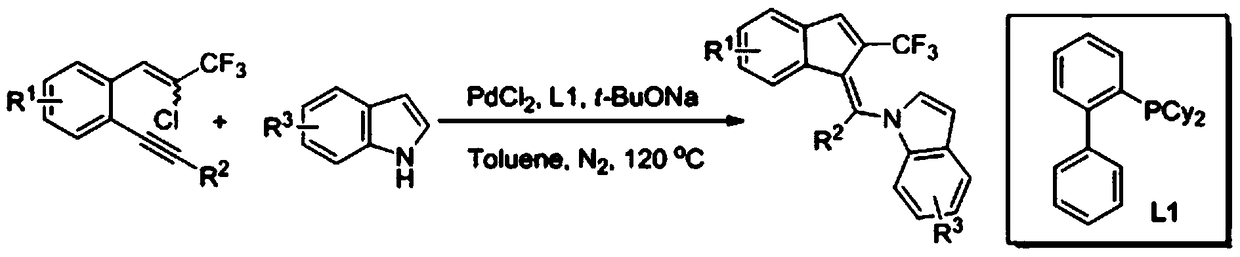
[0019] Specific example one: 0.1mmol of 1-(2-chloro-3,3,3-trifluoroprop-1-en-1-yl)-2-(phenylethynyl)benzene, 0.12mmol of indole , 0.01 mmol of palladium chloride, 0.02 mmol of 2-dicyclohexylphosphobiphenyl, and 0.2 mmol of sodium tert-butoxide were added to 1.0 ml of toluene, and stirred at 120° C. for 24 hours under a nitrogen atmosphere. After the reaction, ethyl acetate was added to the mixture, and washed with saturated brine. After extracting the aqueous layer with ethyl acetate, the combined organic layers were dried over anhydrous sodium sulfate and evaporated in vacuo. The residue was purified by flash column chromatography to give 27.9 mg of yellow solid (Z)-1-(phenyl(2-(trifluoromethyl)-1H-inden-1-ylidene)methyl)-1H-indene Indole, yield 72%, Z / E=98:2, m.p.104-106°C; 1 HNMR (500MHz, CDCl 3 ,TMS)δ:7.65(d,J=8.0Hz,1H),7.50(s,1H),7.47(d,J=7.5Hz,1H),7.41-7.38(m,3H),7.32-7.30(m ,2H),7.16-7.11(m,2H),7.01-7.00(m,1H),6.99-6.96(m,1H),6.94-6.91(m,1H),6.74-6.72(m,2H),6.01 (d...
specific Embodiment 2
[0020] Specific example two: 0.1mmol of 1-(2-chloro-3,3,3-trifluoroprop-1-en-1-yl)-2-(phenylethynyl)benzene, 0.12mmol of 3- Methylindole, 0.01mmol of palladium chloride, 0.02mmol of 2-dicyclohexylphosphobiphenyl, 0.2mmol of sodium tert-butoxide were added to 1.0ml of toluene, and stirred at 120°C for 24 Hour. After the reaction, ethyl acetate was added to the mixture, and washed with saturated brine. After extracting the aqueous layer with ethyl acetate, the combined organic layers were dried over anhydrous sodium sulfate and evaporated in vacuo. The residue was purified by flash column chromatography to give 27.3 mg of (Z)-3-methyl-1-(phenyl(2-(trifluoromethyl)-1H-inden-1-ylidene)methyl as an orange solid )-1H-indole, yield 68%, Z / E=97:3, m.p.121-124°C; 1 H NMR (500MHz, CDCl 3 ,TMS)δ:7.62(d,J=8.0Hz,1H),7.54-7.51(m,2H),7.47-7.43(m,2H),7.39-7.36(m,3H),7.21-7.17(m, 2H),7.02-7.00(m,1H),6.98-6.96(m,1H),6.83(s,1H),6.69(d,J=8.5Hz,1H),6.15(d,J=8.5Hz,1H ),2.37(s,3H); 13 C NMR (...
specific Embodiment 3
[0021] Specific example three: 0.1mmol of 1-(2-chloro-3,3,3-trifluoroprop-1-en-1-yl)-2-(phenylethynyl)benzene, 0.12mmol of 5- Methylindole, 0.01mmol of palladium chloride, 0.02mmol of 2-dicyclohexylphosphobiphenyl, 0.2mmol of sodium tert-butoxide were added to 1.0ml of toluene, and stirred at 120°C for 24 Hour. After the reaction, ethyl acetate was added to the mixture, and washed with saturated brine. After extracting the aqueous layer with ethyl acetate, the combined organic layers were dried over anhydrous sodium sulfate and evaporated in vacuo. The residue was purified by flash column chromatography to give 29.7 mg of (Z)-5-methyl-1-(phenyl(2-(trifluoromethyl)-1H-inden-1-ylidene)methyl as an orange oily liquid )-1H-indole, yield 74%, Z / E=98:2; 1 H NMR (500MHz, CDCl 3 ,TMS)δ:7.53(s,1H),7.52(d,J=7.5Hz,1H),7.46-7.43(m,4H),7.38-7.35(m,1H),7.32-7.31(m,1H) ,7.22-7.19(m,1H),7.01(d,J=3.0Hz,1H),6.99-6.97(m,1H),6.83(d,J=8.5Hz,1H),6.70(d,J=3.0 Hz,1H),6.60(d,J=8.5Hz,1H),6.10(d,J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com