Medical cold compress gel for treating eczematous dermatitis of children and preparation method of medical cold compress gel
A technology for dermatitis, eczema and children, which is applied in the field of preparation of medical cold compress gel, can solve problems such as endocrine system disorder, influence on growth and development, and gastrointestinal discomfort, and achieve the effects of reducing side effects, alleviating inflammation, and alleviating itching.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
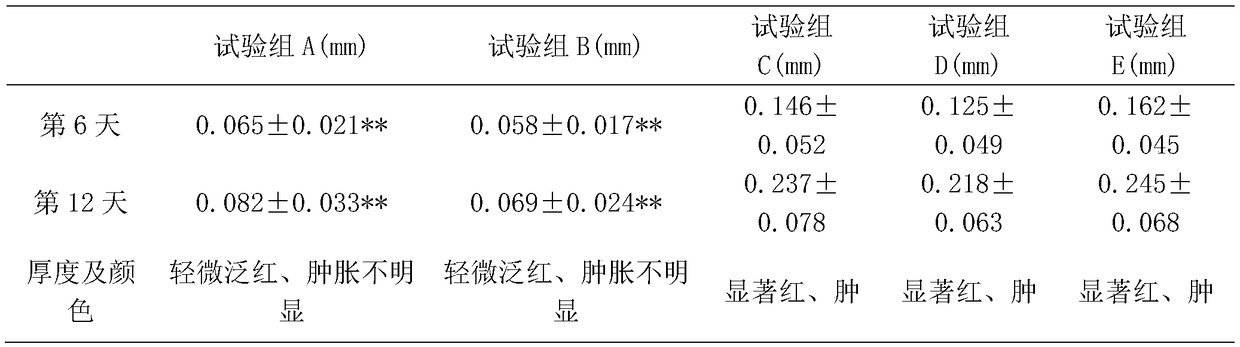
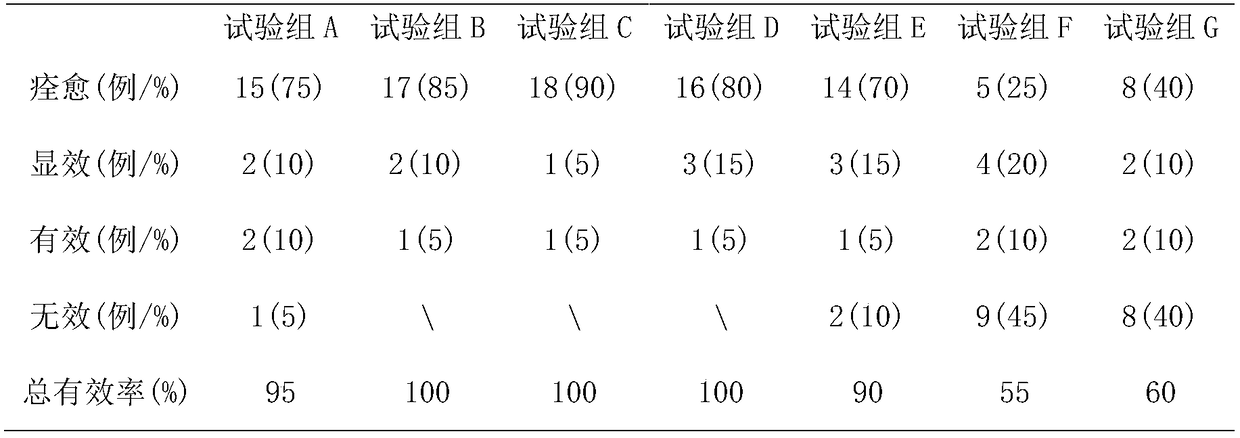
[0034]Preparation steps: Weigh 2g of sun-dried Fangfeng, 20g of lovage, 4g of coix seed, 2g of curcuma, 3g of mint, 1g of perilla leaf and 24g of Tripterygium wilfordii to form component A. Weigh 20g of Xu Changqing, 2g of borneol, 4g of fennel and 5g of cardamom to form component B. Weigh 18g of Schisandra chinensis, 8g of Shijunzi, 6g of Tujingpi and 21g of realgar to form component C. Grind components A, B and C into powder with a traditional Chinese medicine grinder, pass through a 10-mesh sieve, and set aside.
[0035] Preparation of the first extraction extract step: transfer component A to a round-bottomed flask, add 200ml of ether to the round-bottomed flask and mix well, transfer the round-bottomed flask to a constant temperature water bath at 45°C for rotation and reflux extraction for 2.5 hours . After the reflux extraction process ends, the separatory funnel is left standing to separate the medicinal residues and ether, and the ether is transferred to a rotary eva...
Embodiment 2
[0041] Preparation steps: Weigh 4g of sun-dried Fangfeng, 10g of lovage, 12g of coix seed, 7g of curcuma, 8g of mint, 6g of perilla leaf and 16g of Tripterygium wilfordii to form component A. Weigh 8g of Xu Changqing, 10g of borneol, 9g of fennel and 12g of cardamom to form component B. Weigh 8g of Schisandra chinensis, 12g of Shijunzi, 8g of Tujingpi and 16g of realgar to form component C. Grind components A, B and C into powder with a traditional Chinese medicine grinder, pass through a 20-mesh sieve, and set aside.
[0042] Preparation of the first extraction extract step: transfer component A to a round-bottomed flask, add 260ml of ether to the round-bottomed flask and mix well, transfer the round-bottomed flask to a constant temperature water bath at 42°C for rotation and reflux extraction for 2.2 hours . After the reflux extraction process ends, the separatory funnel is left standing to separate the medicinal residues and ether, and the ether is transferred to a rotary ...
Embodiment 3
[0048] Preparation steps: Weigh 5g of sun-dried Fangfeng, 12g of lovage, 10g of coix seed, 5g of curcuma, 7g of mint, 5g of perilla leaf and 13g of Tripterygium wilfordii to form component A. Weigh 11g of Xu Changqing, 7g of borneol, 7g of fennel and 10g of cardamom to form component B. Weigh 12g of Schisandra chinensis, 15g of Shijunzi, 10g of Tujingpi and 13g of realgar to form component C. Grind components A, B and C into powder with a traditional Chinese medicine grinder, pass through a 20-mesh sieve, and set aside.
[0049] Preparation of the first extraction extract step: transfer component A to a round-bottomed flask, add 320ml of ether to the round-bottomed flask and mix well, transfer the round-bottomed flask to a constant temperature water bath at 39°C for rotation and reflux extraction for 2 hours. After the reflux extraction process ends, the separatory funnel is left standing to separate the medicinal residues and ether, and the ether is transferred to a rotary ev...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com