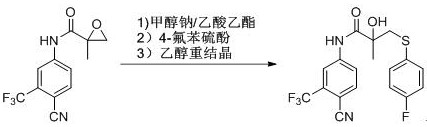
A kind of preparation method of bicalutamide sulfide intermediate
A technology of bicalutamide thioether and intermediates, which is applied in the field of preparation of bicalutamide thioether intermediates, can solve the problems of high cost, long production cycle, cumbersome operation, etc., and achieve simple operation, mild conditions, and reduce cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 200ml of ethyl acetate into a 500ml three-necked bottle, place the three-necked bottle in a high-low temperature cooling and heating machine, cool the temperature of the reaction system to 0-5°C, add 1.0g of sodium methoxide into the three-necked bottle, and then drop Add 10.5 g of 4-fluorothiophenol, continue to stir at this temperature for 0.5 h after the addition, and then add N-[4-cyano-3-(trifluoromethyl)phenyl]-1,2- Epoxy-2-methylpropionamide 20g, after adding, the reaction system was warmed up to room temperature, TLC followed the reaction process, after reacting for 0.5h, the reaction was complete, washed 3 times with saturated brine, and the ethyl acetate was recovered by rotary evaporation under reduced pressure to If it cannot be evaporated, add 10ml of ethanol to dissolve, cool down at 0-5°C to fully crystallize, filter, and air-dry at 60°C to obtain N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[ 25 g of 4-fluorophenylthio]-2-hydroxy-2-methylpropionamide, yie...
Embodiment 2
[0025] Add 200ml of ethyl acetate into a 500ml three-necked bottle, place the three-necked bottle in a high-low temperature cooling and heating machine, cool the temperature of the reaction system to 0-5°C, add 0.25g of sodium methoxide into the three-necked bottle, and then drop Add 10.5 g of 4-fluorothiophenol, continue to stir at this temperature for 0.5 h after the addition, and then add N-[4-cyano-3-(trifluoromethyl)phenyl]-1,2- Epoxy-2-methyl propionamide 20g, after adding, the reaction system was warmed up to room temperature, TLC followed the reaction process, after 36 hours of reaction, the reaction was not complete, washed 3 times with saturated brine, and the ethyl acetate was recovered by rotary evaporation under reduced pressure. If it cannot be evaporated, add 10ml of ethanol to dissolve, cool down at 0-5°C to fully crystallize, filter, and air-dry at 60°C to obtain N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[ 4-fluorophenylthio]-2-hydroxy-2-methylpropionamide 12g, ...
Embodiment 3
[0027] Add 200ml of ethyl acetate into a 500ml three-necked bottle, place the three-necked bottle in a high-low temperature cooling and heating machine, cool the temperature of the reaction system to 0-5°C, add 4.4g of sodium methoxide into the three-necked bottle, and then drop Add 10.5 g of 4-fluorothiophenol, continue to stir at this temperature for 0.5 h after the addition, and then add N-[4-cyano-3-(trifluoromethyl)phenyl]-1,2- Epoxy-2-methylpropionamide 20g, after adding, the reaction system was warmed up to room temperature, TLC followed the reaction process, after reacting for 0.5h, the reaction was complete, washed 3 times with saturated brine, and the ethyl acetate was recovered by rotary evaporation under reduced pressure to If it cannot be evaporated, add 10ml of ethanol to dissolve, cool down at 0-5°C to fully crystallize, filter, and air-dry at 60°C to obtain N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[ 26 g of 4-fluorophenylthio]-2-hydroxy-2-methylpropionamide, yie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com