Method for catalyzing green cyanation of halogenated aromatic hydrocarbons by supported Pd complex
A halogenated aromatic hydrocarbon, supported technology, applied in the field of chemical industry and two-phase catalysis, can solve the problems of strong corrosion, restricting wide application, and difficult post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
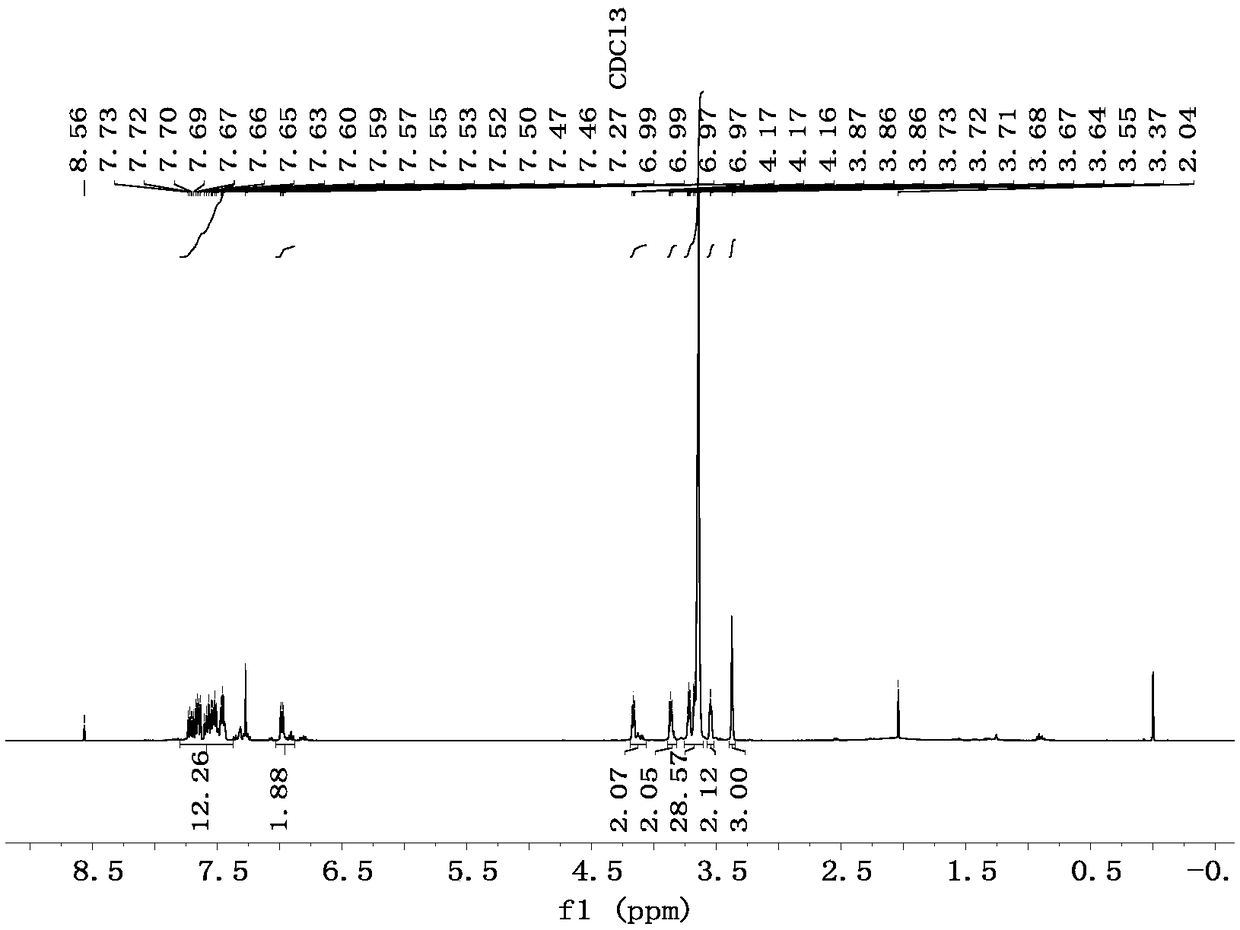
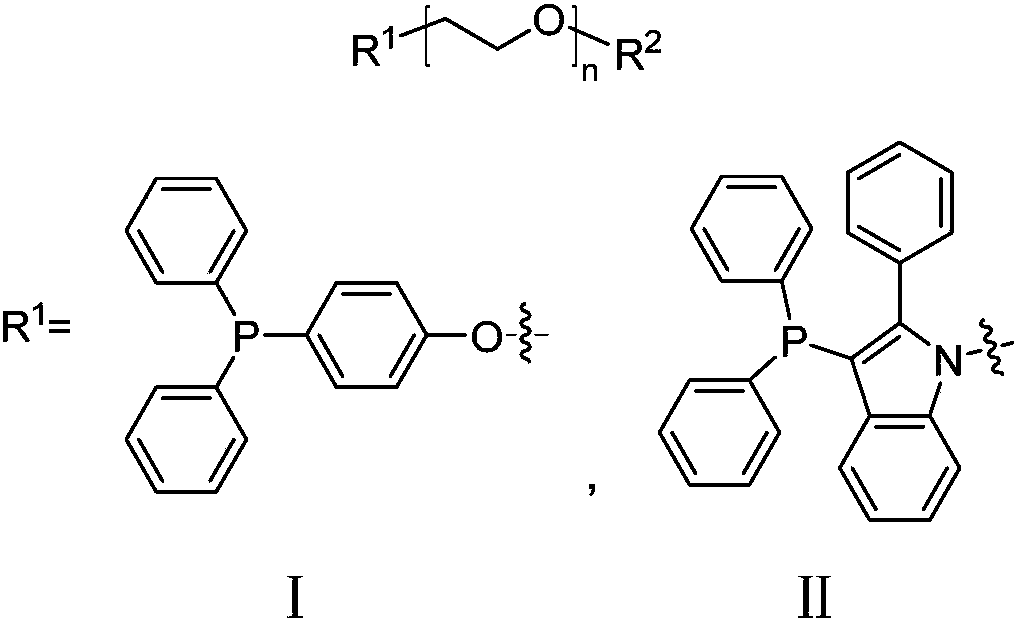
[0034] Preparation of supported ligand I and its method for catalyzing the green cyanation of halogenated aromatic hydrocarbons:
[0035] (1) Preparation of CH 3 O-(CH 2 CH 2 O) 6 -CH 2 CH 2 OTs: CH 3 O-(CH 2 CH 2 O) 6 -CH 2 CH 2 OH (2.5466g, 7.25mmol), NaOH (0.5800g, 20.30mmol) were dissolved in deionized water (15mL) and tetrahydrofuran (15mL), and stirred in an ice-water bath for 0.5h. p-Toluenesulfonyl chloride (TsCl, 1.7273g, 9.06mmol) was dissolved in tetrahydrofuran (20mL) and added dropwise to the reaction system. The stirring reaction was continued for 2h, and then warmed to room temperature for 24h. After the reaction was completed, deionized water (20 mL) was added, extracted with dichloromethane (25 mL×3), the organic phase was washed with saturated brine (15 mL×2), anhydrous Na 2 SO 4 Dry, filter, and remove the organic solvent under reduced pressure to obtain a crude product, which was separated and purified by column chromatography (developing solve...
Embodiment 2
[0041] Preparation of supported ligand Ⅱ and its method for catalyzing the green cyanation of halogenated aromatic hydrocarbons:
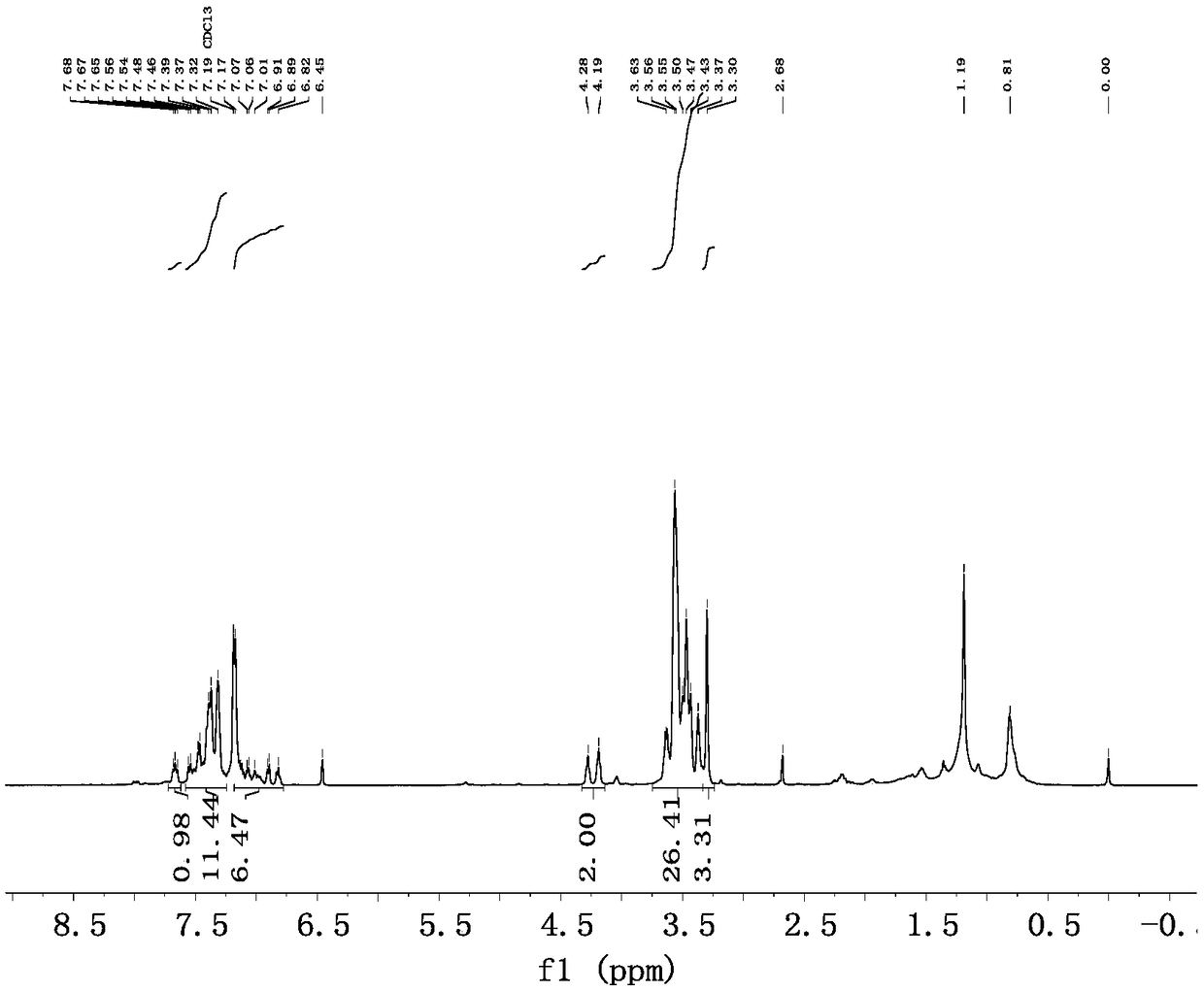
[0042] (1) Preparation of N-(MPEG)-3-bromo-2-phenylindole (MPEG=CH 3 O-(CH 2 CH 2 O) 6 -CH 2 CH 2 -): Under nitrogen protection, 2-phenylindole (868.5mg, 3.19mmol), CH 3 O-(CH 2 CH 2 O) 6 -CH 2 CH 2 OTs (534.4mg, 1.06mmol), NaOH (255.2mg, 6.38mmol) were dissolved in anhydrous DMF (30mL), stirred at 80°C for 48h. After the reaction, DMF was removed by rotary evaporation, deionized water (30 mL) was added, extracted with dichloromethane (20 mL×3), the organic phase was washed with saturated brine (15 mL), anhydrous Na 2 SO 4 Dry, filter, and remove the solvent under reduced pressure to obtain a crude product, which was separated and purified by column chromatography (developing solvent, dichloromethane: anhydrous methanol = 10:1) to obtain 621.1 mg of a yellow-brown oily liquid with a yield of 97%.
[0043] (2) Preparation of Ligand II: ...
Embodiment 3
[0047] Supported Ligand II Catalyzed Recycling Method for Green Cyanylation of Halogenated Aromatics:
[0048] (1) Supported ligand II catalyzes cyanation of halogenated aromatic hydrocarbons: same as step (2) in Example 2.
[0049] (2) will K 4 [Fe(CN) 6 ]·3H 2 O (31.7mg, 0.075mmol) was dissolved in deionized water (2mL) and anhydrous acetonitrile (0.5mL), and added to the reaction system after dissolution, and reacted for 3h. After the reaction, take out the toluene layer while it is hot, and extract the reaction solution twice with toluene (5mL), combine the toluene phases, take out 0.5mL and add anhydrous Na 2 SO 4 Dry, filter, yield 58% by GC. The toluene in the remaining toluene phase was removed by rotary evaporation, isopropanol (2 ml) was added to the residue to dissolve, and extracted with n-hexane. Then the isopropanol was removed by rotary evaporation to obtain the catalyst, and the next catalytic reaction was continued.
[0050] (3) The second cycle method:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com