A kind of roxithromycin capsule and its production process
A technology for roxithromycin and capsules, which is applied in the field of roxithromycin capsules and its production technology, can solve problems such as being unfavorable to pass through the gastrointestinal mucosa, the oil-water distribution coefficient is reduced, and is unstable, so as to improve the relative bioavailability The effect of increasing the solubility, increasing the solubility, and evenly distributing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1, a kind of roxithromycin capsule
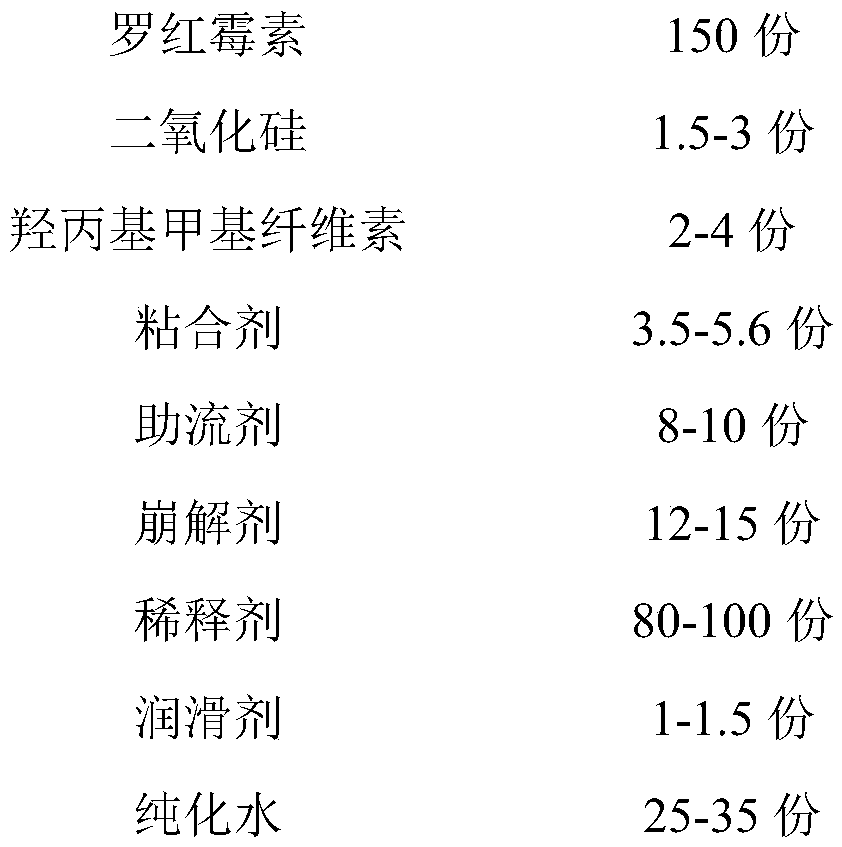
[0035] Described roxithromycin capsule is made up of following composition and content thereof:
[0036]
[0037]
[0038] The adhesive is composed of 2.25kg povidone K30 and 0.27kg polysorbate 80, and the weight ratio is 4.5:0.54.
[0039] Its production process steps are as follows:
[0040] S1. Add povidone K30 into purified water, dissolve and disperse evenly, then add polysorbate 80, disperse evenly, soak in a mixer for 1 hour, stir evenly, and obtain an adhesive;
[0041]S2. Divide roxithromycin, lactose and the adhesive prepared in step S1 into three parts on average, take the first part of roxithromycin and lactose and add them to the granulator, adjust the pressure to 0.4Mpa~1.0Mpa, mix 6min, take the first binder, add it in three times, the binder mass added for the first time and the first total binder mass ratio are 10 / 11, mix and granulate for 2min; The mass ratio of the added binder to the first tota...
Embodiment 2
[0044] Embodiment 2, a kind of roxithromycin capsule
[0045] Described roxithromycin capsule is made up of following composition and content thereof:
[0046]
[0047]
[0048] The adhesive is composed of 1.35kg povidone K30 and 0.225kg polysorbate 80, and the weight ratio is 3.375:0.5625.
[0049] The production technology of described roxithromycin capsule is similar to embodiment 1.
Embodiment 3
[0050] Embodiment 3, a kind of roxithromycin capsule
[0051] Described roxithromycin capsule is made up of following composition and content thereof:
[0052]
[0053] The adhesive is composed of 1.8kg povidone K30 and 0.27kg polysorbate 80, and the weight ratio is 4:0.6.
[0054] The production technology of described roxithromycin capsule is similar to embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com