Levocarnitine thin film coated tablets and preparation method thereof
A technology for film-coated tablets and coated tablets is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, drug delivery, etc., which can solve the problems of unachievable product disintegration, shorten working hours, reduce production costs, and avoid easy moisture absorption. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
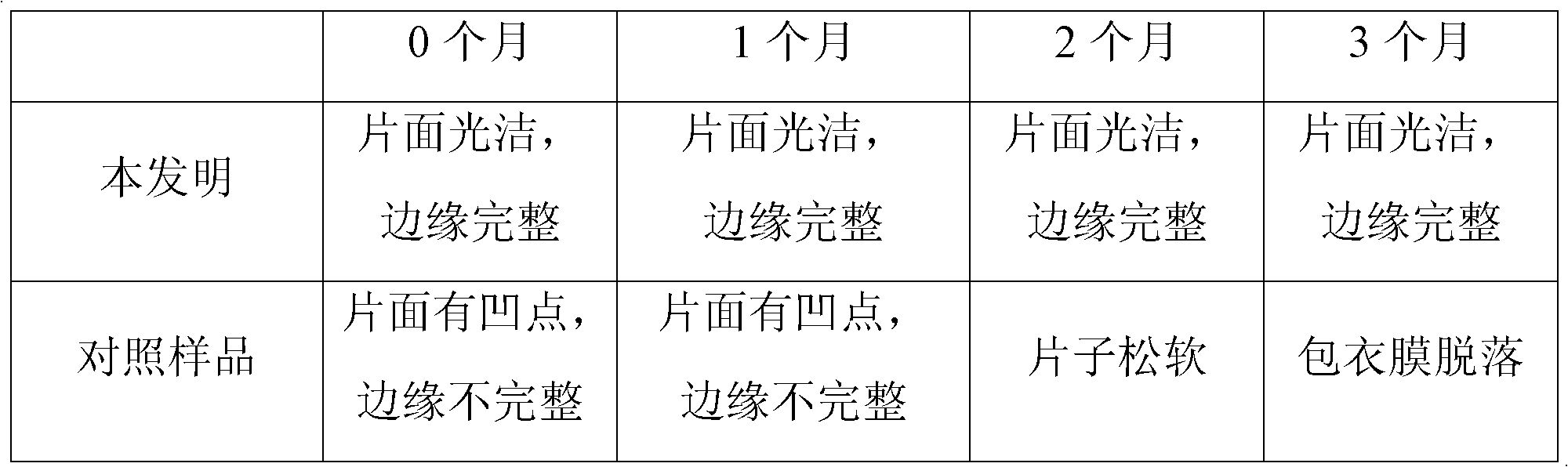
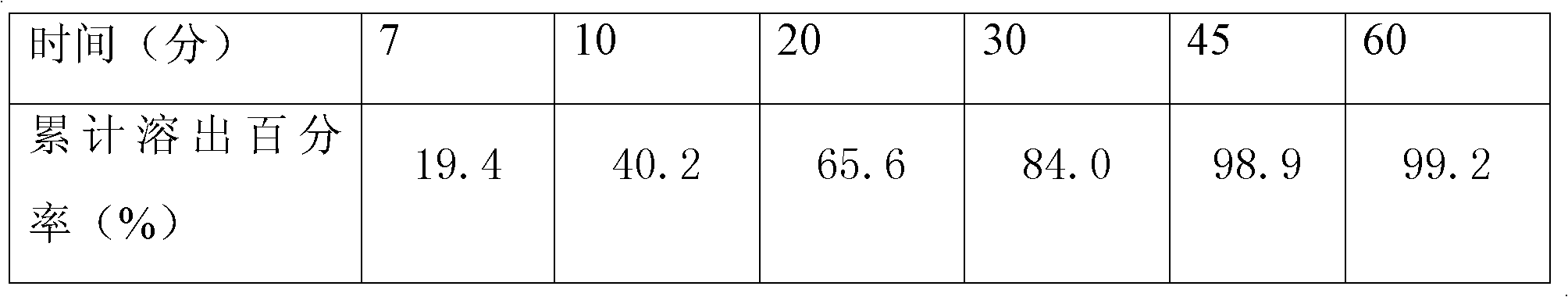
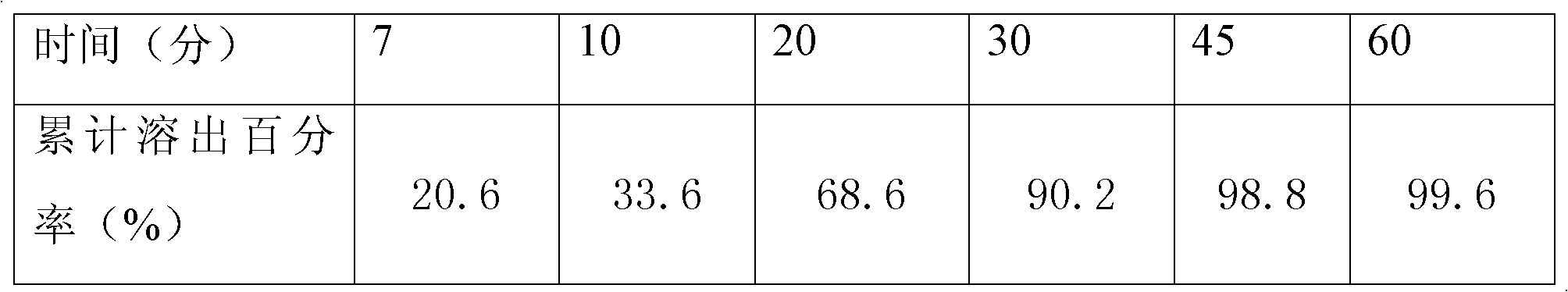
[0016] Example 1: Weigh 330 g of levo-carnitine, 270 g of microcrystalline cellulose, 50 g of lactose, and 6.5 g of silicon dioxide, mix them, add 6.5 g of magnesium stearate, mix them, mix them evenly, and press them into 1000 tablets. The weight is 0.663g, and the tablet hardness is controlled at 6-7Kg; the premix made of 6.6g hydroxypropyl methylcellulose and ethylcellulose film-forming material is dissolved in 76ml of 95% ethanol solution to prepare the isolation layer coating solution, stirred for 45 minutes, sprayed evenly on the surface of levocarnitine tablet core by spray gun, and prepared into levocarnitine isolation gown tablet; dispersing the premix made of 20g polyvinyl alcohol film-forming material in 95ml of distilled water to prepare Moisture-proof layer coating solution, stirred for 45 minutes, uniformly sprayed on the surface of levocarnitine isolation coat tablet through a spray gun; testing and packaging.
Embodiment 2
[0017] Example 2: Weigh 330 g of levocarnitine, 210 g of microcrystalline cellulose, 20 g of pregelatinized starch, 15 g of hydroxypropyl cellulose, 9 g of silicon dioxide, and 20 g of talcum powder for mixing, and add 6 g of magnesium stearate for mixing. Mix evenly and tablet, 1000 tablets, tablet weight 0.61g, tablet hardness control 6-7Kg; 3.1g premix containing ethyl cellulose, polyvinylpyrrolidone film-forming material, dissolved in 95% ethanol solution 36.8ml was prepared as an isolation layer coating solution, stirred for 45 minutes, and evenly sprayed on the surface of levocarnitine tablet core by a spray gun to prepare levocarnitine isolation coating tablet; 12.2g of methacrylic acid copolymer film-forming material was prepared The prepared premix is dispersed in 100ml of distilled water, prepared as a moisture-proof layer coating solution, stirred for 45 minutes, and uniformly sprayed on the surface of the levocarnitine isolation coating tablet by a spray gun; test...
Embodiment 3
[0018] Embodiment 3, take by weighing prescription amount levocarnitine 330g, microcrystalline cellulose 270g, hydroxypropyl cellulose 10g, lactose 15g, silicon dioxide 3.2g, polyethylene glycol 3.2 mix, add magnesium stearate 6.4g mix , mixed evenly, and compressed into 1000 tablets, the tablet weight is 0.638g, and the tablet hardness is controlled at 6-7Kg; the premix made of 9.6g hydroxypropyl methylcellulose film-forming material is dissolved in 95% ethanol solution 111.5 ml to be prepared as an isolation layer coating liquid, stirred for 45 minutes, and evenly sprayed on the surface of levocarnitine tablet cores by a spray gun to prepare levocarnitine isolation coating tablets; The mixed material is dispersed in 73ml of distilled water to prepare a moisture-proof layer coating solution, stirred for 45 minutes, and evenly sprayed on the surface of the levocarnitine isolation coat tablet through a spray gun; tested and packaged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com