Nitrogen-doped super-stable porous polymer composite material and preparation method thereof
A technology of porous polymer and composite material, which is applied in the field of nitrogen-doped ultra-stable porous polymer composite material and its preparation, can solve problems such as poor electrical conductivity, and achieve improved surface wettability, excellent performance, and excellent electrochemical stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
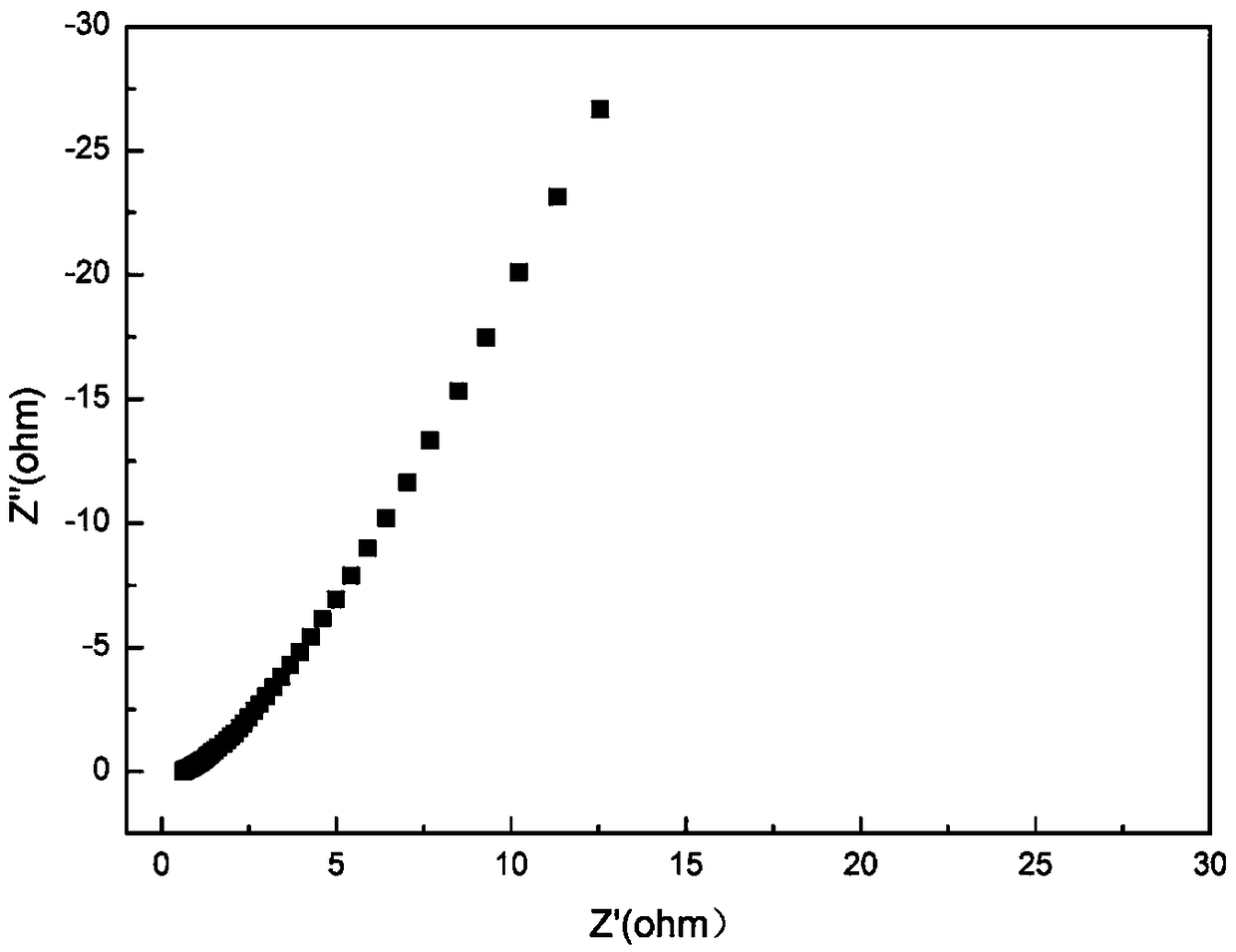
[0025] First prepare the organic ligand, weigh 0.6306g melamine and 2.2501g p-aldehyde benzoic acid and react in 40ml o-xylene at 150°C for 10h. The white product was obtained by suction filtration and purified with water and ethanol 1:1. Vacuum-dried at 80°C for 8 hours, ready for use. Then take 0.2613g organic ligand and 0.2910 cobalt nitrate hexahydrate into the reaction kettle, the solvent is DMF:EtOH=2:1, respectively react at 80°C for 60h, then naturally cool and filter to obtain the product, and dry it at 80°C to obtain Co-PCPs-80 material. Electrochemical performance tests were performed on it, and the charge transfer resistance (Rct) of the composite material was 0.5737Ω., and it had a low equivalent series resistance value and excellent diffusion properties. The cyclic voltammetry curves are rectangular-like with weaker redox peaks, indicating the fast charge propagation capability of both electric double layer capacitance and pseudocapacitance. Calculated from th...
Embodiment 2
[0027] First prepare the organic ligand, weigh 0.6306g melamine and 2.2501g p-aldehyde benzoic acid and react in 40ml o-xylene at 150°C for 9h. The white product was obtained by suction filtration and purified with water and ethanol 1:1. Vacuum-dried at 80°C for 8 hours, ready for use. Then take 0.2613g of organic ligand and 0.2910g of cobalt nitrate hexahydrate into the reaction kettle, the solvent is DMF:EtOH=2:1, respectively react at 100°C for 60h, then naturally cool and filter to obtain the product, and dry it at 80°C to obtain Co-PCPs-100 material. Electrochemical performance tests were carried out on it. The charge transfer resistance (Rct) of the composite material was 0.6319Ω., and it had a low equivalent series resistance value and excellent diffusion performance. The cyclic voltammetry curves are rectangular-like with weaker redox peaks, indicating the fast charge propagation capability of both electric double layer capacitance and pseudocapacitance. Calculated ...
Embodiment 3
[0029] First prepare the organic ligand, weigh 0.6306g melamine and 2.2501g p-aldehyde benzoic acid and react in 50ml o-xylene at 155°C for 10h. The white product was obtained by suction filtration and purified with water and ethanol 1:1. Vacuum-dried at 80°C for 8 hours, ready for use. Then take 0.2613g organic ligand and 0.2910 cobalt nitrate hexahydrate into the reaction kettle, the solvent is DMF:EtOH=1:1, respectively react at 120°C for 50h, then naturally cool and filter to obtain the product, and dry it at 80°C to obtain Co-PCPs-120 material. The electrochemical performance test shows that the charge transfer resistance (Rct) of the composite material is 0.562Ω., and has a low equivalent series resistance value and excellent diffusion performance. The cyclic voltammetry curves are rectangular-like with weaker redox peaks, indicating the fast charge propagation capability of both electric double layer capacitance and pseudocapacitance. Calculated from the charge-disch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com