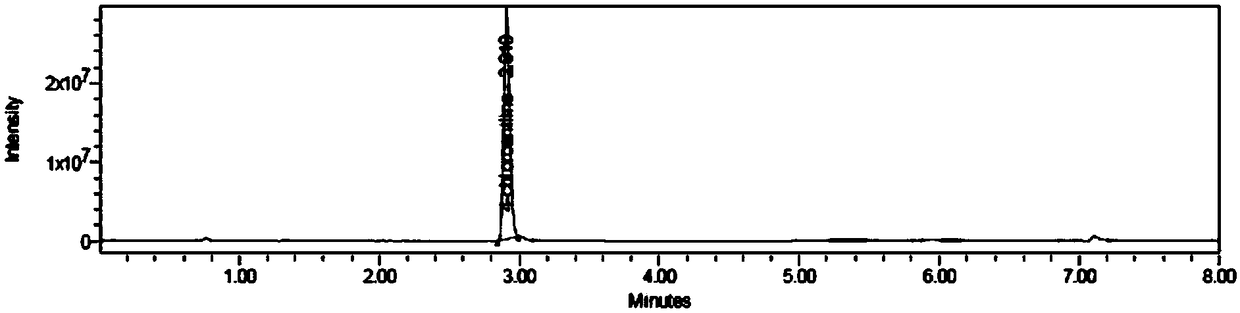
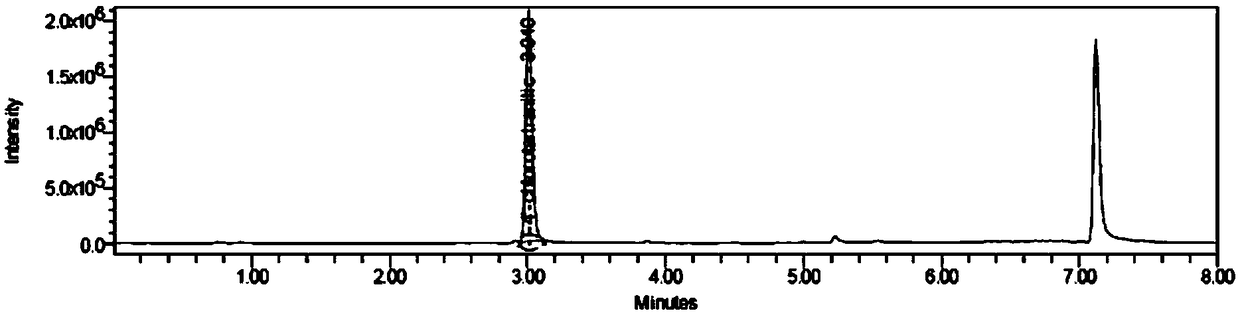
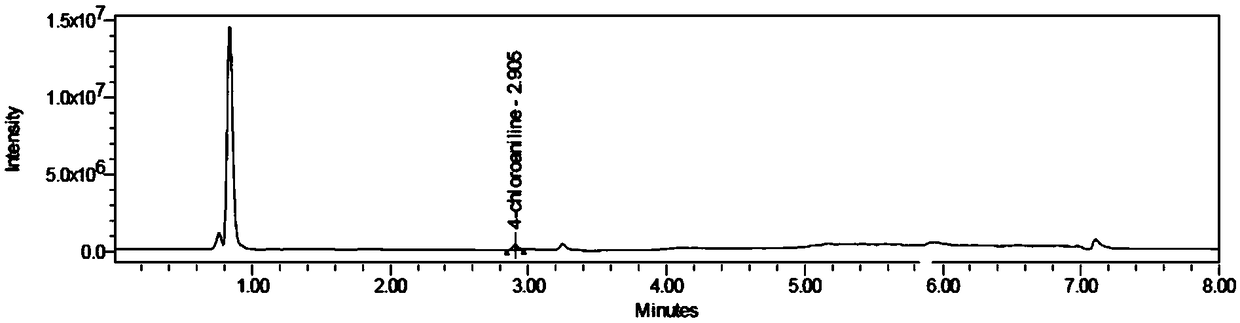
Method for determining aniline-type genotoxic impurities in novel solifenacin succinate crude drug
A technology of Solidium Sodium and raw materials, which is applied in the field of drug testing of Solidium Succinate New Raw Materials, to achieve the effect of reducing the use of organic solvents, reducing environmental hazards, and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A method for the determination of genotoxic impurities in a new raw material drug of sodium succinate, including the following steps:
[0041] S1. Prepare 4-chloroaniline and (-)-4'-chloroaniline wine with 4-chloroaniline and (-)-4'-chloroaniline tartaramic acid as the reference substance and 50% acetonitrile aqueous solution as the solvent Paraffinic acid reference substance solution, take the new sodium succinate raw material drug, and dissolve it with 50% acetonitrile aqueous solution to obtain the test solution;
[0042] S2. Prepare two 4-chloroaniline and (-)-4'-chloroaniline tartaramic acid reference solutions in parallel, and obtain 4-chloroaniline and (-)- in the reference solution by UPLC-MS method. The peak area of 4'-chloroaniline tartaramic acid, calculate the ratio of the peak area to the concentration of the reference solution, and obtain the response factors of 4-chloroaniline and (-)-4'-chloroaniline tartaramic acid;
[0043] S3. Take the test solution to de...
Embodiment 2
[0058] The linear range of the measurement method provided in Example 1 was investigated.
[0059] Take 10mg of 4-chloroaniline and (-)-4'-chloroaniline tartaramic acid in a 10mL volumetric flask, dissolve and dilute to the mark with 50% acetonitrile, shake well, as the reference substance stock solution 1; accurately measure Put 300μL of reference substance stock solution 1 in a 100mL volumetric flask, dilute to the mark with 50% acetonitrile, shake well, and serve as reference substance stock solution 2;
[0060] Pipette 0.05mL, 0.25mL, 0.4mL, 0.5mL, 0.75mL, 1mL of the reference substance stock solution 2 respectively into a 10mL volumetric flask, dilute to the mark with 50% acetonitrile, shake well, and mark them as L-10 , L-50, L-80, L-100, L-150 and L-200. Pipette 0.1 mL of L-100 into a 10 mL volumetric flask, dilute to the mark with 50% acetonitrile, and shake up to make L-1%.
[0061] According to the measurement method provided in Example 1, samples were injected and determ...
Embodiment 3
[0065] The standard addition recovery test of the determination method provided in Example 1.
[0066] The accuracy of the measurement method provided in Example 1 was analyzed by the method of standard addition and recovery.
[0067] Take 10 mg of the new raw material of solitary sodium succinate, accurately weigh it, and place 11 parts in parallel in a 10 mL volumetric flask. Take 2 parts of them and dissolve them with 50% acetonitrile to the mark, mix well, and determine 4 in the sample. -Chloroaniline and (-)-4'-Chloroaniline tartaramic acid content; take 3 of them, add 0.25mL reference substance stock solution 2, dissolve it with 50% acetonitrile to the mark, and mix well, as 50% Add standard recovery solution; take 3 of them, add 0.5mL reference substance stock solution 2, dissolve to the mark with 50% acetonitrile, mix well, as a 100% standard recovery solution; take 3 of them and add 0.7 mL of reference substance stock solution 2, dissolved in 50% acetonitrile to the mark,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume ratio | aaaaa | aaaaa |
| volume ratio | aaaaa | aaaaa |
| volume ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com