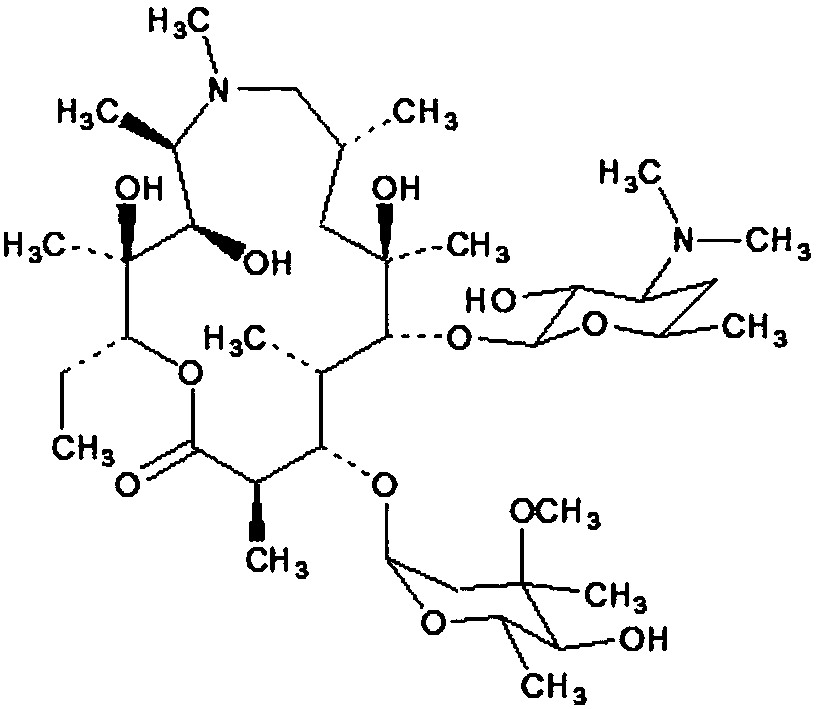
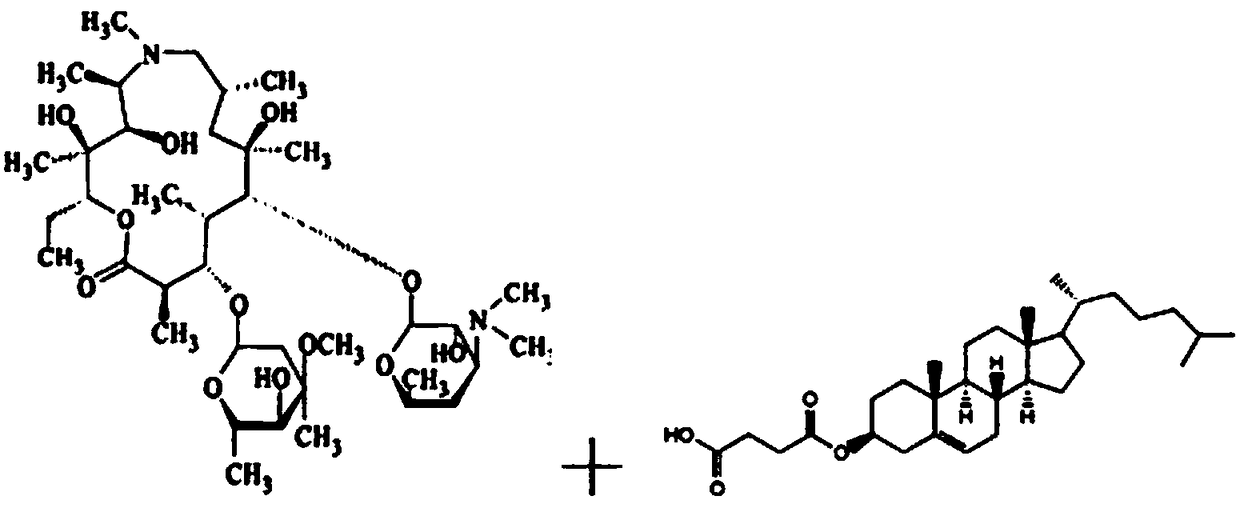
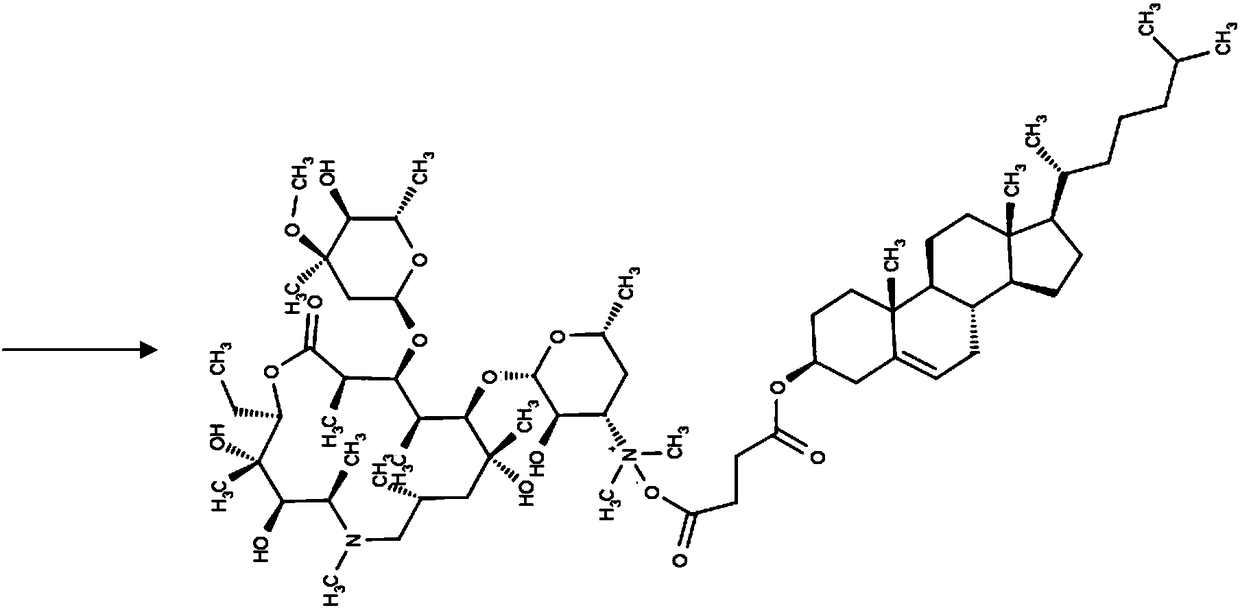
Azithromycin ion pair liposome eye drops and preparation method thereof
A technology of azithromycin and liposome, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as insoluble azithromycin oil
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Formula 1 Azithromycin content 1g: 100ml
[0051] Based on 100ml eye drops
[0052]
[0053] The preparation steps of azithromycin ion-pair liposome eye drops are as follows:
[0054] Step 1: Na 2 HPO 4 12H 2 O 0.29g, NaH 2 PO 4 2H 2 O 0.13g, KH 2 PO 4 Disperse 0.02g, NaCl0.8g, KCl0.02g in 100mL water for injection, dissolve them all, and prepare the water phase, keep it warm at 60°C, and set aside;
[0055] Step 2: Dissolve azithromycin 1g, egg yolk lecithin PC-98T 8g, cholesterol succinate monoester 2g, DSPE-PEG 20000.1g, dl-α-tocopherol 0.05g, medium chain triglyceride 8.5g in chloroform and transfer into a round-bottomed flask, and chloroform was removed by rotary evaporation to obtain a yellow oily liquid. Continue drying the obtained oily substance under nitrogen flow for 1 h; hydrate the obtained oily substance with the aqueous phase prepared in step 1 at 60° C. for 30 minutes to obtain azithromycin liposome coarse dispersion system, which...
Embodiment 2
[0060] Example 2 formula 2 azithromycin content 2g: 100ml
[0061] Based on 100ml eye drops
[0062]
[0063] The preparation steps of azithromycin ion-pair liposome eye drops are the same as in Example 1.
[0064] Among them, the liposome has no oil droplets, no insoluble components or massive aggregates under the naked eye observation, no azithromycin precipitation under the microscope, the particle size is 137.0nm, the Zeta potential is -28.36mv, the pH value is 7.79, and the content is 93.4%. The encapsulation rate is 92.7%, and the osmotic pressure is in the range of 280-310 mosm / kg.
Embodiment 3
[0065] Example 3 Formula 3 Azithromycin content 0.5g: 100ml
[0066] Based on 100ml eye drops
[0067]
[0068] The preparation steps of azithromycin ion-pair liposome eye drops are the same as in Example 1.
[0069] Among them, the liposome has no oil droplets, no insoluble components or massive aggregates under the naked eye observation, no azithromycin precipitation under the microscope, the particle size is 29.8nm, the Zeta potential is -19.64mv, the pH value is 7.88, and the content is 99.6%. The encapsulation rate is 98.3%, and the osmotic pressure is in the range of 280-310 mosm / kg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com