Transdermal drug delivery system of high-solubility ibuprofen or structural analog of ibuprofen
A drug delivery system and a technology similar in structure, applied in the field of transdermal drug delivery systems of high-solubility ibuprofen or its structural analogs, can solve the problems of large patch area, unpracticalability, low content and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] A transdermal drug delivery system for ibuprofen with high solubility, comprising a polymer matrix layer, the polymer matrix layer comprising active ingredient ibuprofen, HPMCAS, a second polymer compound and a pressure-sensitive adhesive; the second The polymer compound is a pharmaceutically acceptable fat-soluble polymer and / or amphiphilic polymer.
[0091] Further, the ibuprofen transdermal drug delivery system of this embodiment also includes a backing layer and a protective layer; the polymer matrix layer is located between the backing layer and the protective layer.
[0092] In this embodiment, the weight content of ibuprofen in the polymer matrix layer is 30%, the weight content of the pressure-sensitive adhesive is 35%, the weight content of HPMCAS is 15%, and the weight content of the second polymer compound is 15%; Pharmaceutically acceptable excipients (such as talcum powder, colloidal SiO 2 , montmorillonite, vitamin E, etc.).
[0093] In this embodiment, ...
experiment example 1
[0101] Experimental Example 1 Stability Experiment
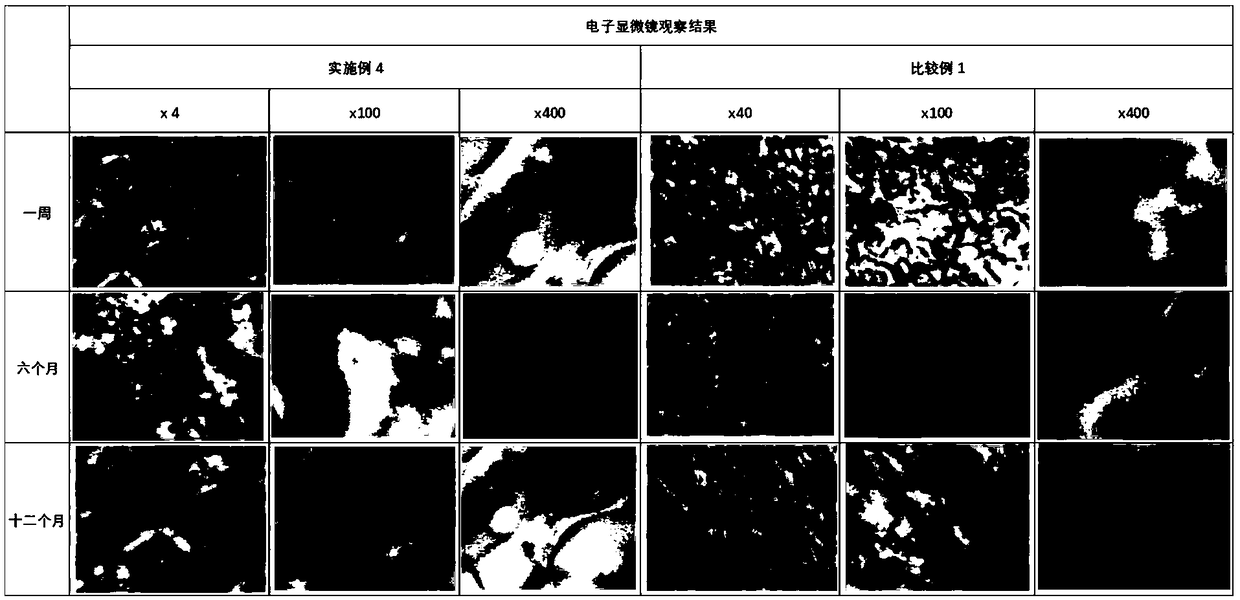
[0102] The ibuprofen delivery system (patch) prepared in Comparative Example 1 and Example 4 is placed under the same storage conditions (30 ± 2° C., 60% ± 10% RH), and the polymer matrix is regularly observed by an electron microscope The surface condition, the observation results see figure 1 .
[0103] Continuous observation results show that both Comparative Example 1 and Example 4 can be stored stably. The types of polymers contained in the polymer matrix are different, and the morphology of the polymer matrix is also different. In Comparative Example 1 where only HPMCAS is contained in the polymer matrix, the surface of the polymer matrix is uniformly distributed, while in Example 4 where both HPMCAS and ethylcellulose EC are contained in the polymer matrix, the polymer matrix appears as clusters. Different types and different proportions of polymers lead to different aggregation states of the polymer matrix, ...
experiment example 2
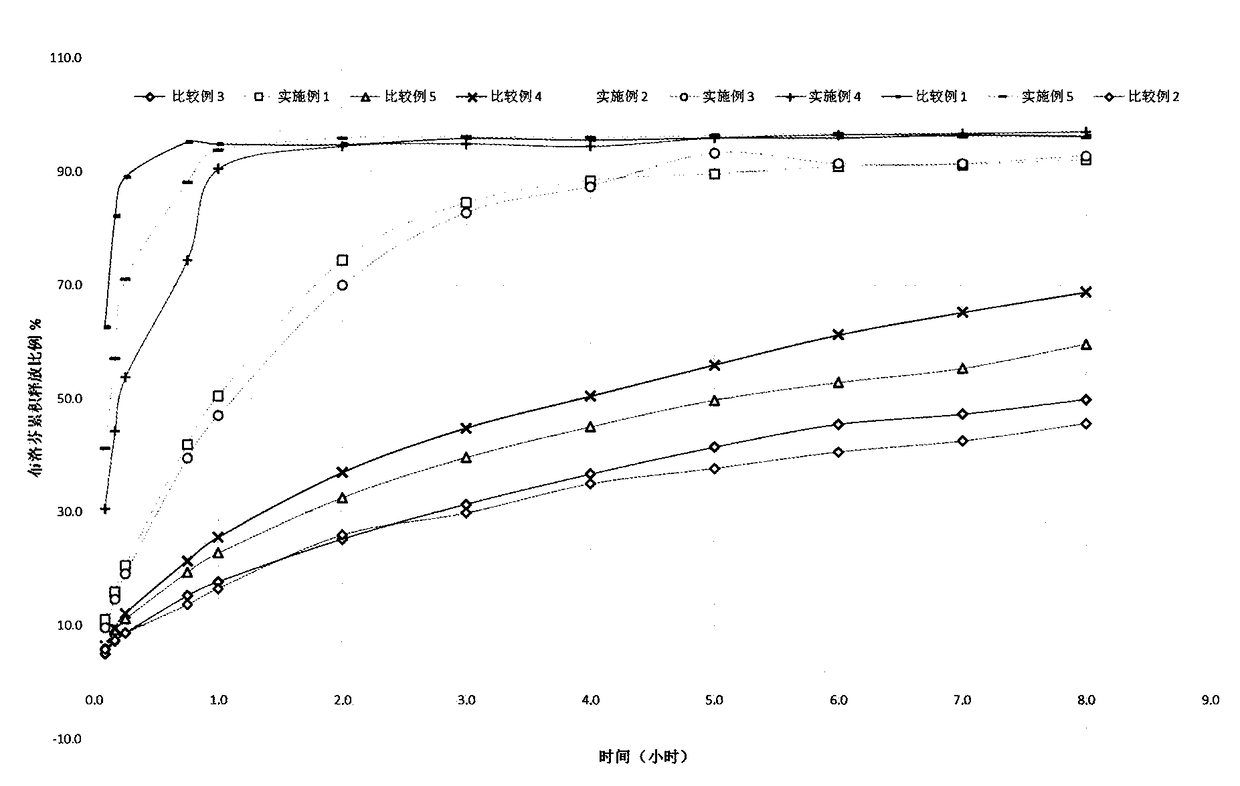
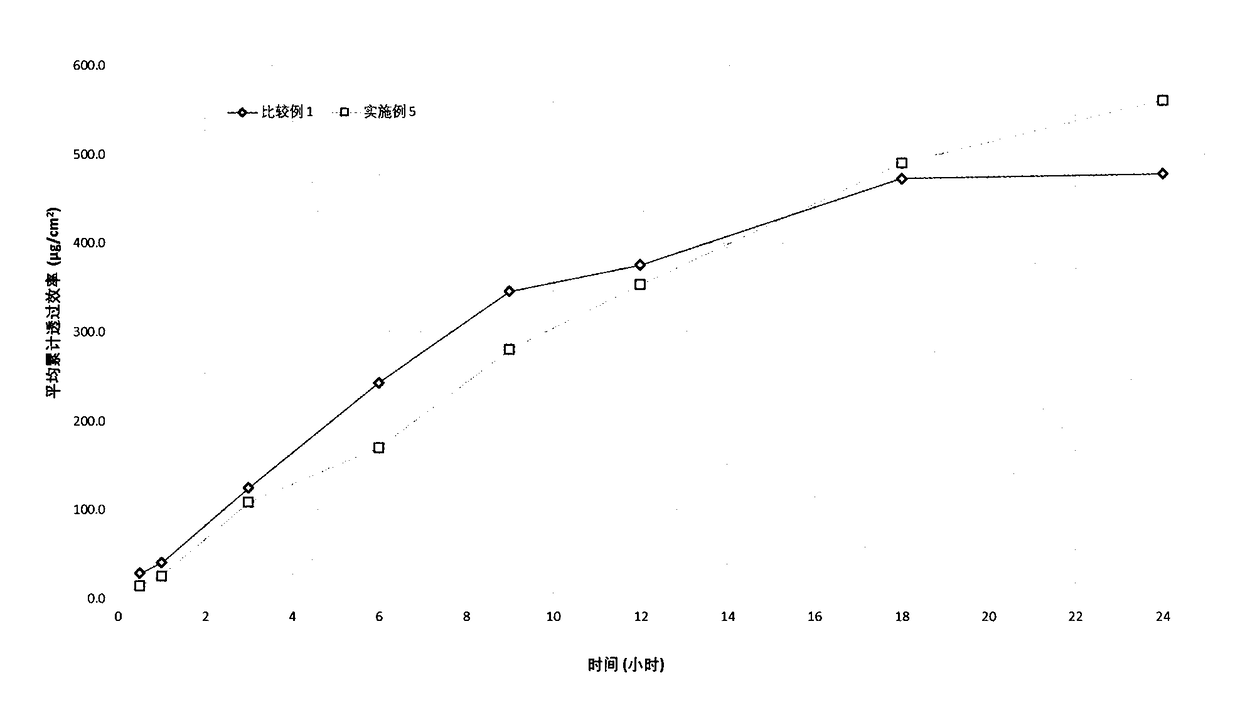
[0104] Experimental example 2 in vitro release test
[0105] In vitro release is a basic performance index of the patch, reflecting the interaction between the active ingredient and other components in the polymer matrix. The overall properties of the polymer matrix, the interactions between ibuprofen and the polymer and other components such as hydrogen bonds, ion pairs, van der Waals forces, etc., lead to different behaviors of ibuprofen flowing in the polymer matrix. In vitro release is the basis of transdermal absorption, and only appropriate release capacity can meet specific transdermal absorption requirements.
[0106]Dissolution determination method (Chinese Pharmacopoeia 2015 Edition 4th General Rule 0931 4th Method - paddle-disc method), using PBS as the dissolution medium, the temperature is 32°C, 50 rpm, and it is operated according to the law. Take 10ml samples at 0.1h, 0.2h, 0.3h, 0.8h, 1.0h, 2.0h, 3.0h, 4.0h, 5.0h, 6.0h, 7.0h and 8.0h, filter; and accurately we...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com