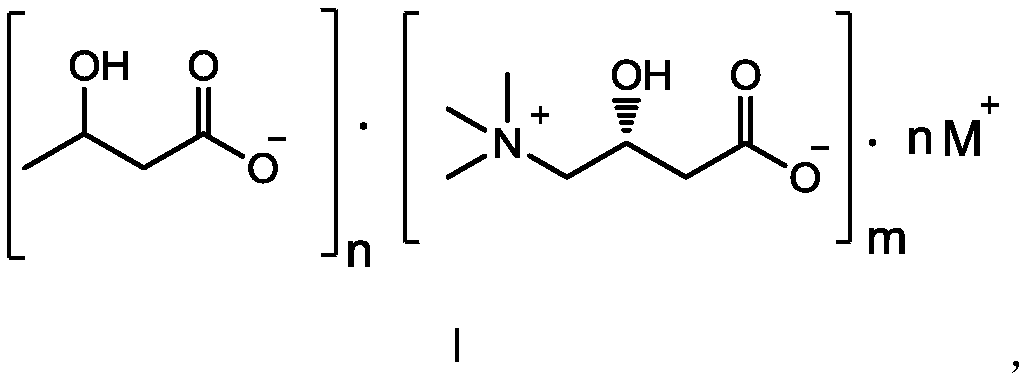
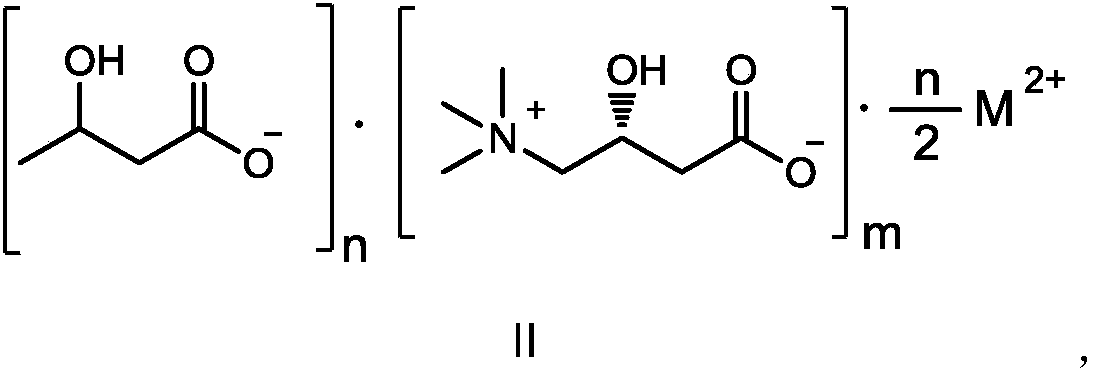
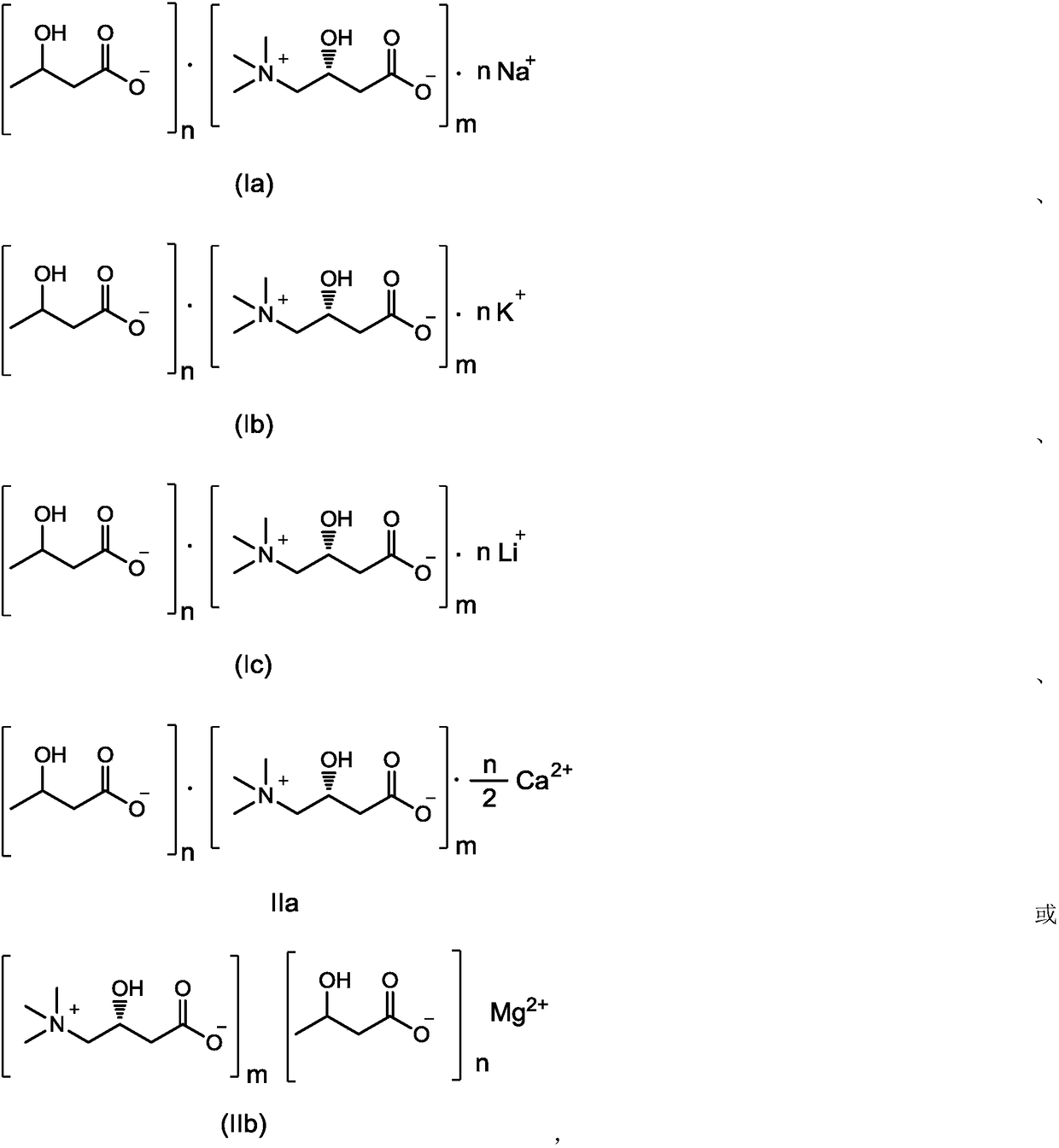
Composition containing L-carnitine and beta-hydroxybutyric acid compounds
A technology of hydroxybutyric acid histidine and hydroxybutyric acid, applied in the field of medicine, can solve the problems of expensive, ineffective, mineral balance imbalance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The preparation method of embodiment 1, compound (Iaa):
[0065]
[0066] 1.04 g (10 mmol) of 3-hydroxybutyric acid, 8 mL of acetone were added to a 100 mL round bottom flask. Dissolve 1.61g (10mmol) of L-carnitine in 3mL of pure water, and add 1mL of 10N (10mmol) aqueous sodium hydroxide solution into the solution. Slowly drop the prepared L-carnitine solution into the reaction flask, and react for 5 minutes after the dripping is completed, remove the solvent from the reaction solution in a water bath at 40°C under reduced pressure to obtain a colorless oil, add 80 mL of acetone to the flask, Stir and crystallize, filter, and wash the filter cake with acetone and vacuum-dry to obtain 2.58 g of a white solid, with a yield of 90%.
[0067] Chemical Formula: C 11 h 22 NO 6Na; 1H NMR (D 2 O): δ1.12(3H,d),2.12~2.41(4H,m),3.14(9H,s),3.34(2H,dd),4.04~4.09(1H,m),4.47~4.50(1H, m).
Embodiment 2
[0068] Embodiment 2: the preparation of following formula compound (IIaa)
[0069]
[0070] 1.04 g (10 mmol) of 3-hydroxybutyric acid, 16 mL of acetone were added to a 100 mL round bottom flask. Dissolve 1.61g (10mmol) L-carnitine in 16mL pure water, add 0.37g (5mmol) calcium hydroxide powder to the solution, stir and heat to 80°C for 2 hours, then drop the suspended L-carnitine solution Put it into a reaction flask, heat it to 60°C and stir for 2 hours, and then dissolve it. Remove the solvent under reduced pressure in a water bath at 40°C to obtain a milky white oil. Add 80 mL of acetone to the flask, stir and crystallize, filter, and filter cake After washing with acetone and vacuum drying, 1.76 g of white product was obtained, with a yield of 86%.
[0071] Chemical Formula: C 22 h 44 N 2 o 12 Ca; 1 HNMR (D 2 O): δ1.13(3H,d),2.11~2.40(8H,m),3.15(18H,s),3.36(4H,dd),4.05~4.13(2H,m),4.47~4.51(2H, m).
Embodiment 3
[0072] Example 3 Preparation of L-carnitine-β-hydroxybutyrate arginine (wherein the molar ratio of L-carnitine to β-hydroxybutyrate arginine is 1:1): add 2.78g to a 100mL round bottom flask (11 mmol) β-hydroxybutyrate arginine, add 10 mL of acetone and 3 mL of purified water. Dissolve 1.61g (10mmol) of L-carnitine in 3mL of pure water, and add 1mL of 10N (10mmol) sodium hydroxide solution into the solution. Slowly drip the prepared L-carnitine solution into the reaction flask. After the dripping, react for 5 minutes. Remove the solvent from the reaction solution in a water bath at 40°C under reduced pressure to obtain a colorless oil. Add 80 mL of acetone to the flask. After stirring for 20 minutes, a white solid precipitated out, which was filtered, and the filter cake was washed with acetone and then vacuum-dried to obtain 3.92 g of a white product, with a yield of 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com