Novel rare-earth-free porous fluorescent material and preparation method thereof
A fluorescent material and rare earth-free technology, applied in the field of fluorescent materials, can solve the problems of complex preparation process, narrow excitation spectrum, non-adjustment and the like, and achieve the effects of simple preparation process, wide excitation spectrum and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
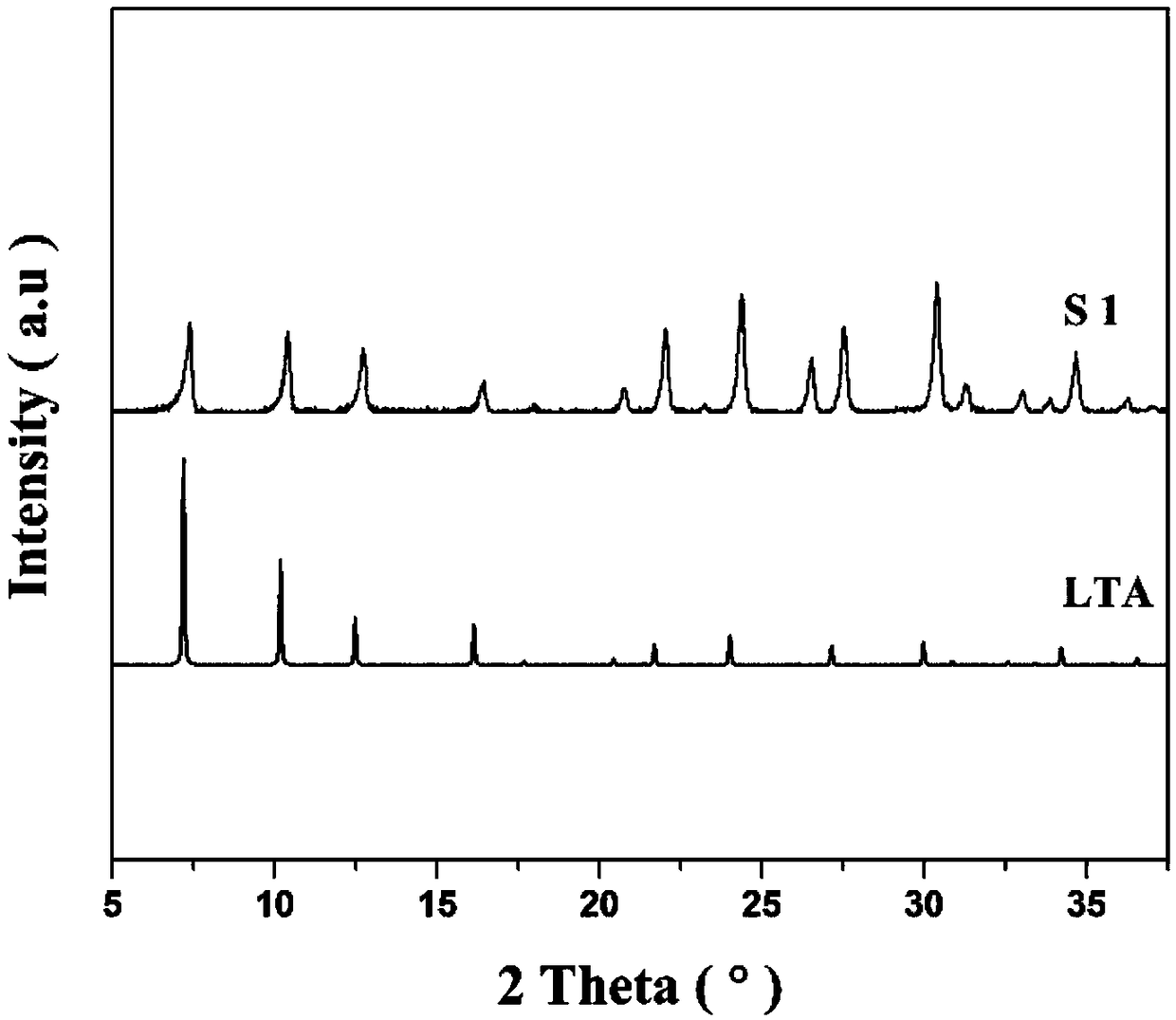
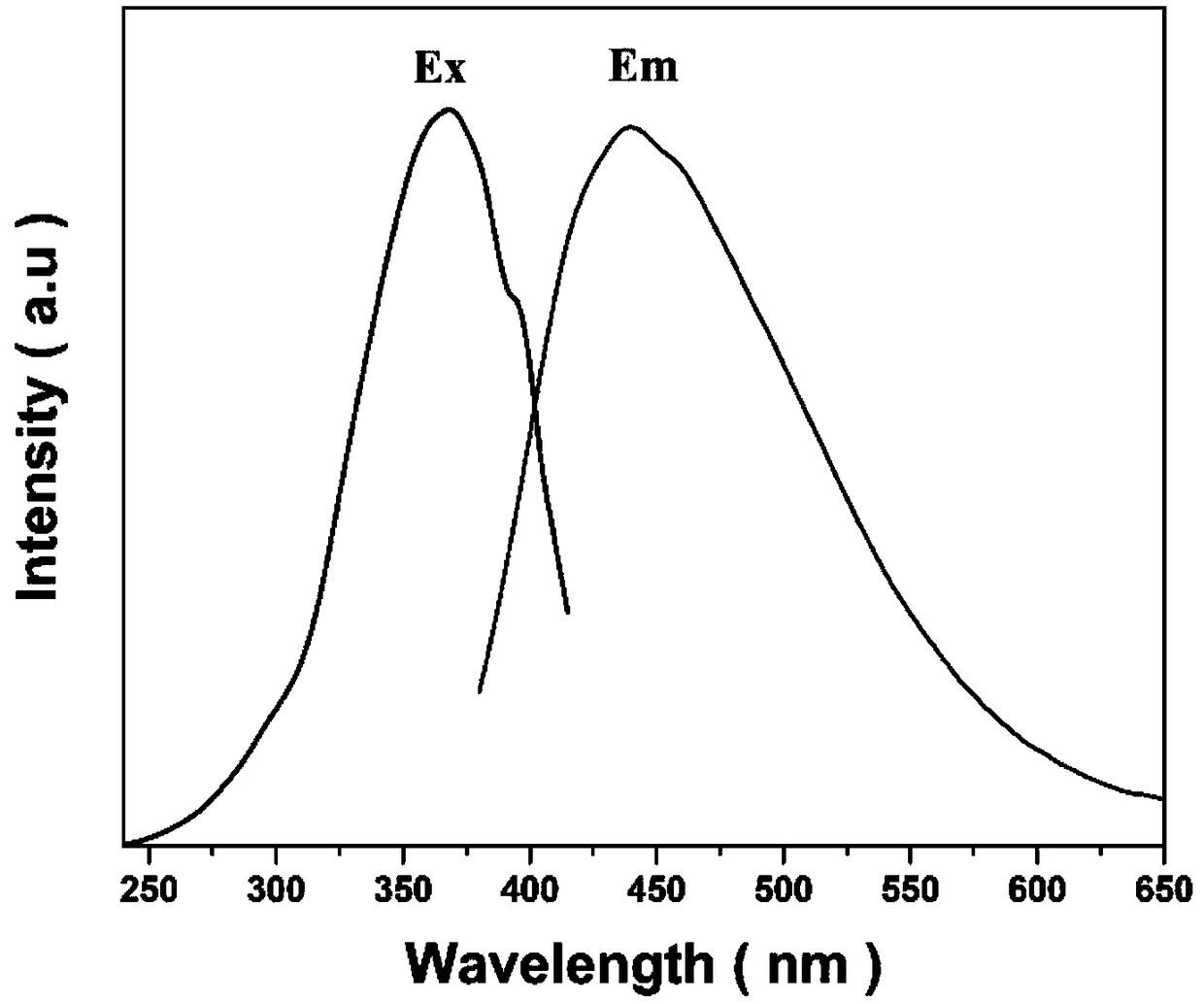
[0029] Weigh 1.2g of silicic acid, 1.0g of aluminum isopropoxide, 13.30g of TMAOH (25wt%) solution and 164μL of NaOH (10M) according to the molar ratio of each component in the chemical formula, and dissolve them in 6.9g of deionized water to completely dissolve the raw materials into the solution clarify. Put the mixed solution in a 50mL airtight container, put it in an oven at 70°C, and react for 2 days, wash the obtained precipitate with deionized water several times until the pHfigure 1 As shown, S1 is the XRD pattern of the fluorescent material obtained in Example 1, and LTA in the figure is the standard diffraction pattern of the LTA zeolite crystal. Such as figure 2 As shown, the fluorescent material obtained in Example 1 can be excited by ultraviolet light and emit blue light with a peak wavelength of 435 nm.
Embodiment 2
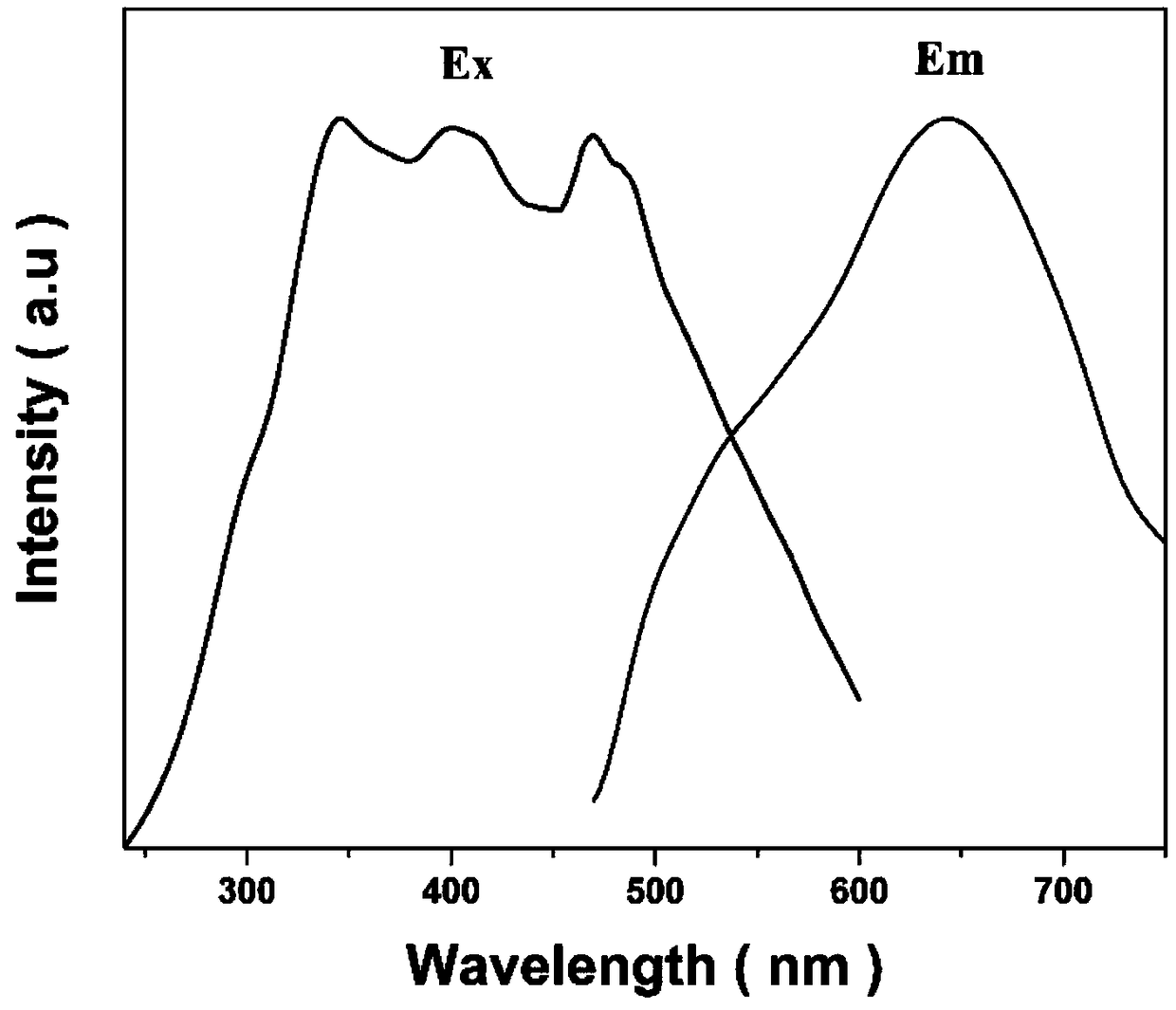
[0031] Weigh 1.2g of silicic acid, 1.0g of aluminum isopropoxide, 13.30g of TMAOH (25wt%) solution and 164μL of NaOH (10M) according to the molar ratio of each component in the chemical formula, and dissolve them in 6.9g of deionized water to completely dissolve the raw materials into the solution clarify. Put the mixed solution in a 50mL airtight container, put it in an oven at 70°C, and react for 2 days, wash the obtained precipitate with deionized water several times until the pH image 3 As shown, the obtained fluorescent material can be effectively excited by a wide band of ultraviolet to blue light, and emit red light with a peak wavelength of 640nm. Experiments have proved that by adjusting the temperature and time of sintering, the emission wavelength can fluctuate in the range of 530nm-640nm, and the scheme suitable for different products can be easily obtained through parameter setting.
Embodiment 3
[0033] Take by weighing 1.2g silicic acid, 1.0g aluminum isopropoxide, 13.30gTMAOH (25wt%) solution, 1.02gAPS and 164μL NaOH (10M) according to the molar ratio of each component in the chemical formula, be dissolved in 6.9g deionized water, make raw material completely Dissolve until the solution is clear. Put the mixed solution in a 50mL airtight container, put it in an oven at 70°C, and react for 3 days, wash the obtained precipitate with deionized water several times until the pH Figure 4 As shown, the obtained fluorescent material samples are monodisperse uniform spherical nanoparticles. Such as Figure 5 As shown, the obtained fluorescent material is a microporous material, and its specific surface area is calculated to be 515m 2 / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com