Metal iridium catalyst with 2, 2'-bis-benzimidazole ligand and method for synthesizing N-methylated primary amine
A technology of bisbenzimidazole and synthesis method, applied in the directions of organic compound/hydride/coordination complex catalyst, chemical instrument and method, physical/chemical process catalyst, etc., can solve problems such as long reaction time, and achieve reaction conditions Mild, broad development prospects, high reaction atom economy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
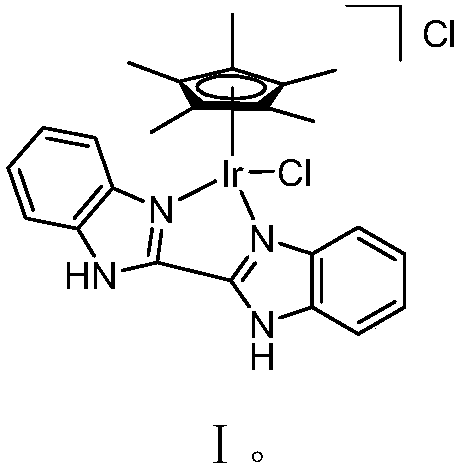
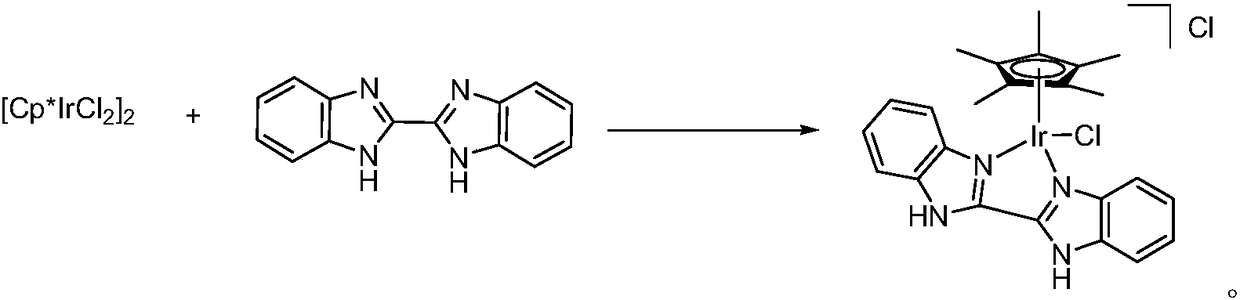
[0033] [Cp*Ir(BiBzImH2)Cl][Cl]
[0034]
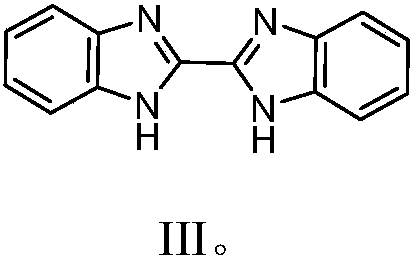
[0035] Dichloro(pentamethylcyclopentadienyl) iridium dimer (100mg, 0.126mmol), bisbenzimidazole (65mg, 0.277mmol, 2.2equiv) and N,N-dimethylformamide (3mL ) into a 25mL Kirschner tube in turn. Under nitrogen protection, react at a temperature of 60° C. for 12 hours, and then cool to room temperature. Filtration and washing with petroleum ether gave the target product as a yellow solid. Yield: 65%
[0036] 1 H NMR (500MHz, CDCl 3 )δ14.9(br s,2H),7.78(d,J=8.4Hz,2H),7.70(d,J=8.4Hz,2H),7.50(t,J=7.2Hz,2H),7.45(t ,J=7.2Hz,2H),1.84(s,15H); 13 C NMR (125MHz, CDCl 3 )δ144.1,138.9,134.2,126.2,125.1,116.5,115.0,87.6,10.2.HRMS-EI(70eV)m / zcalcd for C 24 h 25 ikB 4 [M+H] + 597.1392, found 597.1397.
[0037] Its single crystal structure is as follows:
[0038]
[0039] Single crystal structure: bond length (10 -10 m), bond angle (degrees): Ir-N1, 2.145(11); Ir-N3, 2.117(13); Ir-C5(Cp*), 2.159(16); Ir-Cl, 2.400(5); N1 -Ir-N3, 75.9...
Embodiment 2
[0041] N-Methylaniline
[0042] N-methylbenzonamine
[0043]
[0044] Aniline (46 mg, 0.5 mmol), cat 1 (3.2 mg, 0.005 mmol, 1 mol%), cesium carbonate (49 mg, 0.15 mmol, 0.3 equiv.) and methanol (1 mL) were sequentially added to a 25 mL Kirschner tube. After reacting at a temperature of 120° C. for 12 hours, it was cooled to room temperature. Rotary evaporation removes solvent, then obtains pure target compound by column chromatography (developing solvent: sherwood oil / ethyl acetate), productive rate: 92%
[0045] 1HNMR (500MHz, CDCl 3 )δ7.19(t, J=7.7Hz, 2H, ArH)), 6.7(t, J=7.4Hz, 1H, ArH), 6.6(d, J=8.1Hz, 2H, ArH), 2.8(s, 2H,CH 3 ); 13 C NMR (125MHz, CDCl 3 ) δ149.2, 129.1, 117.1, 112.3, 30.6.
Embodiment 3
[0047] 4-Chloro-N-methylaniline
[0048] 4-chloro-N-methylbenzonamine
[0049]
[0050] p-Chloroaniline (71 mg, 0.5 mmol), cat 1 (3.2 mg, 0.005 mmol, 1 mol%), cesium carbonate (49 mg, 0.15 mmol, 0.3 equiv.) and methanol (1 mL) were sequentially added to a 25 mL Kirschner tube. After reacting at a temperature of 120° C. for 12 hours, it was cooled to room temperature. Rotary evaporation removes solvent, then obtains pure target compound by column chromatography (developing solvent: sherwood oil / ethyl acetate), productive rate: 90%
[0051] 1 HNMR (500MHz, CDCl 3 )δ7.11(d, J=8.8Hz, 2H, ArH), 6.51(d, J=8.8Hz, 2H, ArH), 3.69(br s, 1H, NH), 2.79(s, 3H, CH 3 ); 13 C NMR (125MHz, CDCl 3 ) δ 147.8, 128.8, 121.5, 113.5, 30.6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com