Preparation method for high-purity isocaprylic acid
A technology of isooctanoic acid and high purity, applied in the field of preparation of isooctanoic acid, can solve the problems of low product purity, poor quality, difficult removal of 2-ethylhexenoic acid, etc., and achieve the effect of simple process and high product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
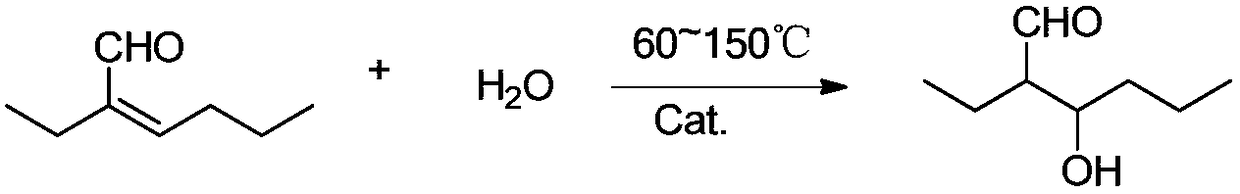
[0035] 2-Ethylhexanal hydrogenation solution obtained by condensation and hydrogenation of n-butyraldehyde, a total of 100g, wherein the content of 2-ethyl-2-hexenal is 0.1wt%, 2-ethyl-3-hydroxyhexanal Content 0.1wt%, 2-ethylhexanal content 90wt%, other 9.8wt%. The hydrogenation solution was mixed with 2g of water, added to the reaction kettle, mixed with 2g of acid clay powder catalyst, the reaction temperature was 100°C, and reacted for 30min, and the catalyst powder was separated from the reaction solution to obtain a mixture solution.
[0036] In a dry 1L three-neck flask, add 80g of the above-mentioned 2-ethylhexanal mixture, place it in a water bath, and stir it mechanically under a nitrogen atmosphere. After the temperature rises to 30°C, start to introduce air with a flow rate of 11.6g / h, keep the reaction temperature at 30-35°C by adding cooling water to the water bath, and react at normal pressure for 8 hours to obtain the 2-ethylhexanoic acid oxidation reaction sol...
Embodiment 2
[0038] 2-Ethylhexanal hydrogenation solution obtained by condensation and hydrogenation of n-butyraldehyde, a total of 100g, wherein the content of 2-ethyl-2-hexenal is 0.5wt%, 2-ethyl-3-hydroxyhexanal Content 0.2wt%, 2-ethylhexanal content 91wt%, other 8.3wt%. The hydrogenation solution is mixed with 5g of water, and sent into a tubular reactor filled with 10mL cation exchange resin, and the space velocity of the hydrogenation solution mixture is 1.0h -1 , the reaction temperature is 130°C, and a mixture solution is obtained after the reaction.
[0039] In a dry 1L three-neck flask, add 80g of the above-mentioned 2-ethylhexanal mixture, place it in a water bath, and stir it mechanically under a nitrogen atmosphere. After the temperature rises to 30°C, start to introduce air with a flow rate of 12.2g / h, keep the reaction temperature at 30-35°C by adding cooling water to the water bath, and react at normal pressure for 6 hours to obtain the 2-ethylhexanoic acid oxidation reac...
Embodiment 3
[0041] 2-Ethylhexanal hydrogenation liquid obtained by condensation and hydrogenation of n-butyraldehyde, a total of 100g, wherein the content of 2-ethyl-2-hexenal wt1%, the content of 2-ethyl-3-hydroxyhexanal 0.3wt%, 2-ethylhexanal content 92.5wt%, other 6.2wt%. The hydrogenation solution was mixed with 8g of water, and sent into a tubular reactor filled with 10mL acidic alumina particle catalyst. The space velocity of the hydrogenation solution mixture was 2.0h-1, and the reaction temperature was 150°C. After the reaction, a mixture solution was obtained.
[0042] According to the method of Example 1, the mixture was oxidized by air to obtain the 2-ethylhexanoic acid oxidation reaction solution, which was subjected to vacuum distillation and separation, and the water and 2-ethyl-3-hydroxyhexanoic acid were first separated under normal pressure. The azeotrope was separated by rectification under reduced pressure. The pressure was 5000 Pa (gauge pressure), the reflux ratio was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com