Method for synthesizing alpha-keto ester by dehydrogenation coupling reaction of 8-methyl quinoline derivatives and alpha-keto acid under palladium catalysis
A technology of methylquinoline and dehydrogenation coupling, applied in the field of synthesis of α-keto ester derivatives, to overcome the effects of high toxicity of reaction reagents, mild reaction conditions and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
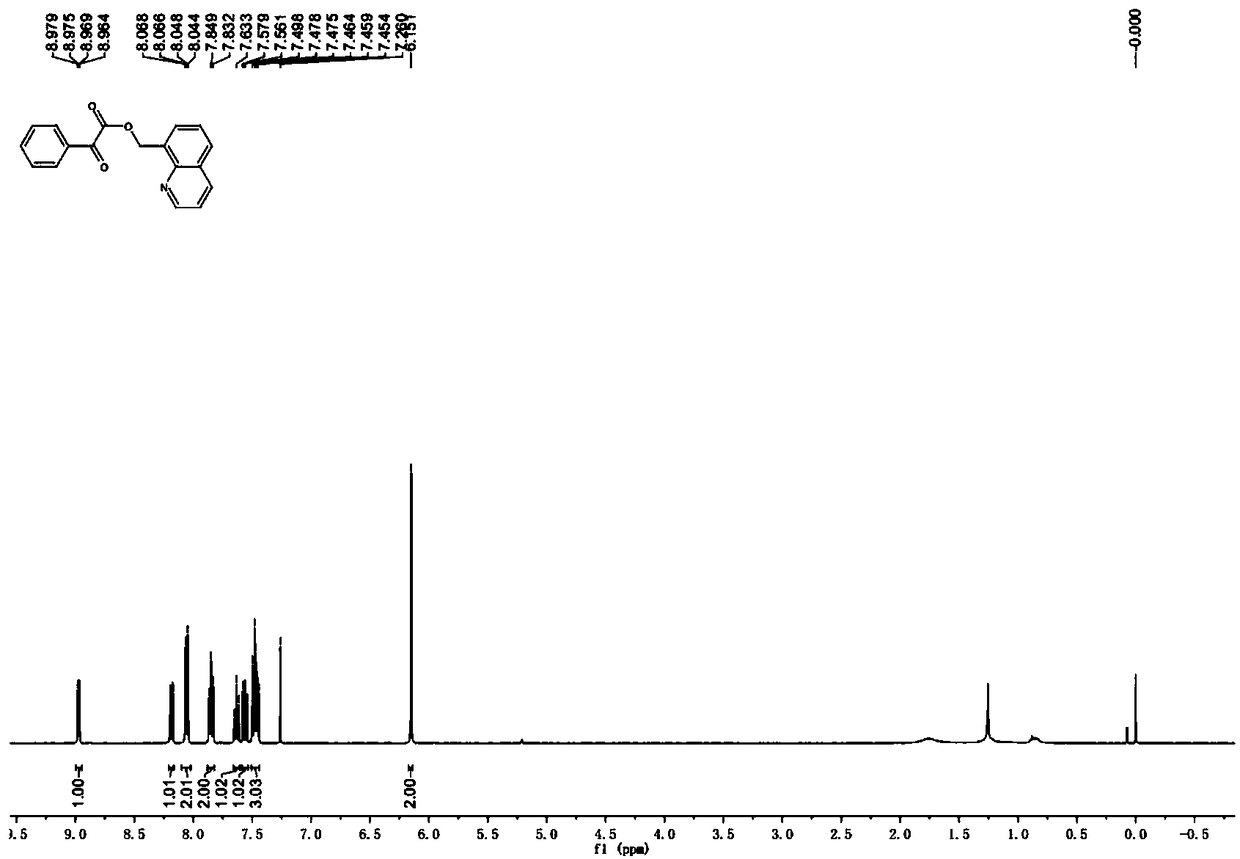
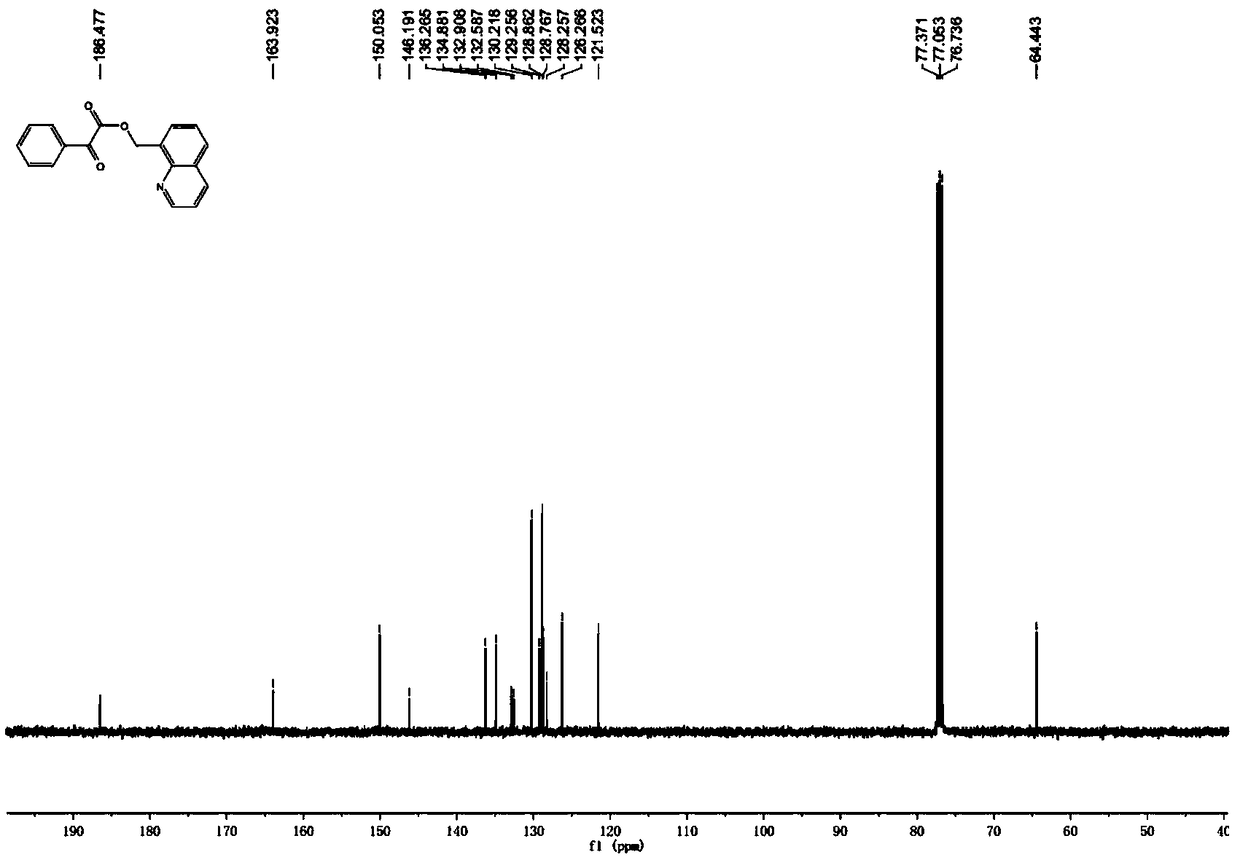
[0035] 8-Methylquinoline (14.3 mg, 0.1 mmol), benzoic acid (30.0 mg, 0.2 mmol), tris(dibenzylideneacetone)dipalladium (9.2 mg, 0.01 mmol), iodobenzene diacetate ( 80.5mg, 0.25mmol), and a stirring bar into the reaction tube, add 1 ml of solvent CHCl 3 , seal the reaction tube. The reaction tube was placed in a 70°C oil bath reaction pot and stirred for 12 hours. After cooling to room temperature, it was neutralized with saturated sodium bicarbonate solution and extracted with ethyl acetate. The extracts were combined and dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure. The crude product was subjected to column chromatography with ethyl acetate: petroleum ether=1:4 (volume ratio) as the eluent to obtain pure product. White solid, melting point 71-72°C, yield 80%. 1 H NMR (400MHz, CDCl 3 )δ8.97(dd,J=4.2,1.8Hz,1H),8.18(dd,J=8.2,1.8Hz,1H),8.07-8.04(m,2H),7.85(t,J=6.6Hz,2H) ), 7.65-7.62(m, 1H), 7.56(dd, J=8.2, 7.0Hz, 1H),...
Embodiment 2
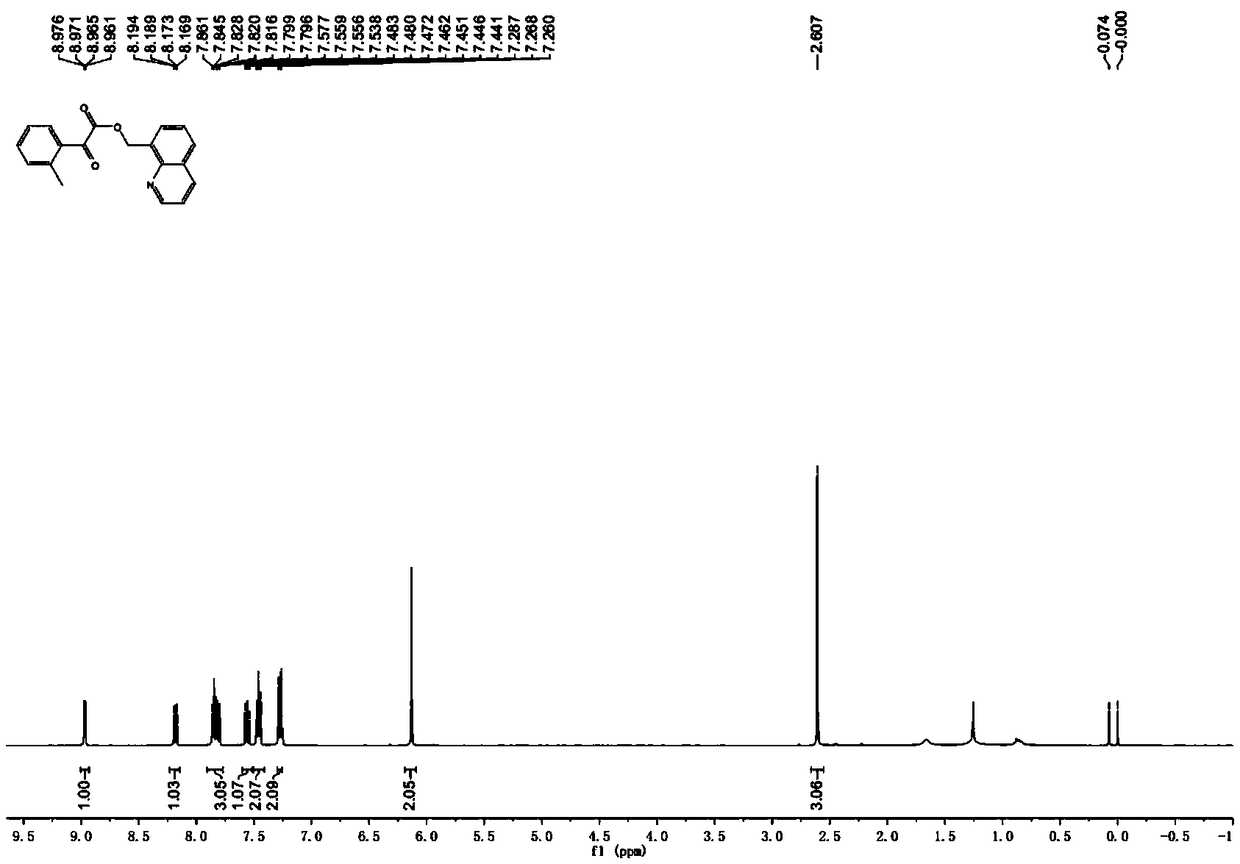
[0037] 7,8-Dimethylquinoline (15.7 mg, 0.1 mmol), benzoylformic acid (30.0 mg, 0.2 mmol), tris(dibenzylideneacetone)dipalladium (9.2 mg, 0.01 mmol), diacetic acid Iodobenzene (80.5mg, 0.25mmol) and a stirring bar were put into the reaction tube, and 1 ml of solvent CHCl was added 3 , seal the reaction tube. The reaction tube was placed in a 70°C oil bath reaction pot and stirred for 12 hours. After cooling to room temperature, it was neutralized with saturated sodium bicarbonate solution and extracted with ethyl acetate. The extracts were combined and dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure. The crude product was subjected to column chromatography with ethyl acetate: petroleum ether=1:4 (volume ratio) as the eluent to obtain pure product. White solid, mp 118-119°C, yield 71%. 1 H NMR (400MHz, CDCl 3 )δ8.96(s,1H),8.12(d,J=8.0Hz,1H),8.03(d,J=7.6Hz,2H),7.76(d,J=8.4Hz,1H),7.60(t, J=7.2Hz, 1H), 7.46-7.37(m, 4H), 6...
Embodiment 3
[0039] 6,8-Dimethylquinoline (15.7 mg, 0.1 mmol), benzoylformic acid (30.0 mg, 0.2 mmol), tris(dibenzylideneacetone)dipalladium (9.2 mg, 0.01 mmol), diacetic acid Iodobenzene (80.5mg, 0.25mmol) and a stirring bar were put into the reaction tube, and 1 ml of solvent CHCl was added 3 , seal the reaction tube. The reaction tube was placed in a 70°C oil bath reaction pot and stirred for 12 hours. After cooling to room temperature, it was neutralized with saturated sodium bicarbonate solution and extracted with ethyl acetate. The extracts were combined and dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure. The crude product was subjected to column chromatography with ethyl acetate: petroleum ether=1:4 (volume ratio) as the eluent to obtain pure product. Yellow oil, 75% yield. 1 H NMR (400MHz, CDCl 3 )δ8.90(dd,J=4.0,1.6Hz,1H),8.10-8.05(m,3H),7.68-7.60(m,3H),7.48(t,J=7.8Hz,2H),7.41(dd , J=8.2, 4.2Hz, 1H), 6.11(s, 2H), 2.54(s,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com