The use of grass aconitine
A kind of clathrin, the technology of external preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
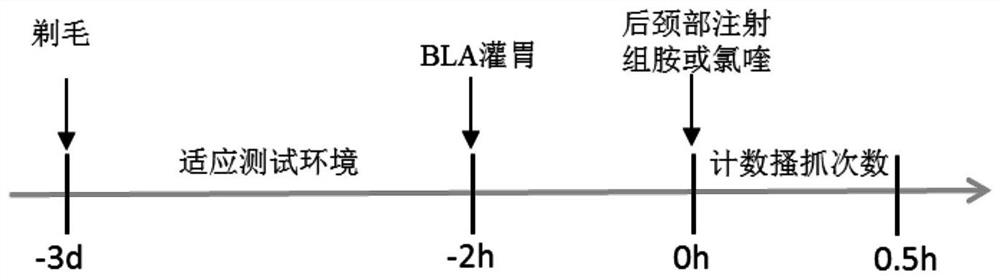
[0034] Purpose:
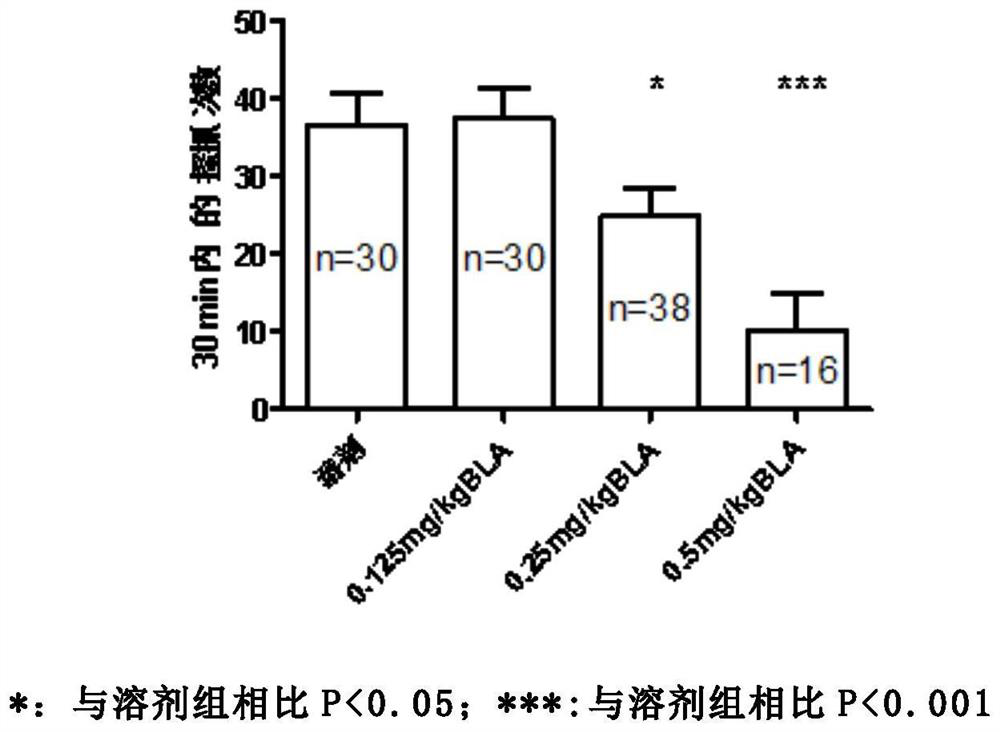
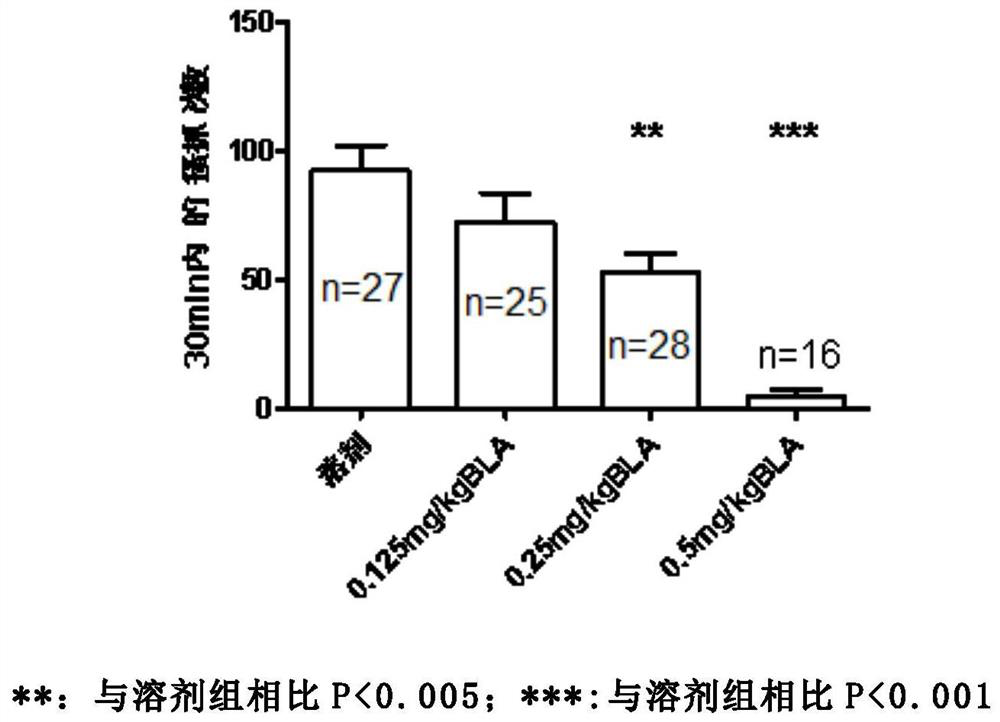
[0035] Studies over the years have shown that itching is mainly conducted by two types of C fibers, one sensitive to histamine and the other sensitive to chloroquine. Therefore, the animal scratching response induced by subcutaneous injection of histamine or chloroquine is an internationally recognized model of acute pruritus. The purpose of the present invention is to explore whether orthoacine (BLA) can inhibit the acute itching caused by histamine and chloroquine.
[0036] Experimental materials and methods:
[0037]6-8 week C57 mice (body weight 17-20 g, the animals were obtained from the Experimental Animal Center of Sun Yat-Sen University) were used in the experiment. The mice were randomly divided into four groups: solvent+histamine control group, BLA+histamine group, solvent+chloroquine control group and BLA+chloroquine group. Three days before the start of the experiment, the nape of the neck was shaved (at least 3 x 5 cm) under brief anesthesia w...
Embodiment 2
[0053] The application of the aconitin of the present invention in neuropathic pruritus can make the aconitin into different dosage forms for use. To be specific, aconitin can be made into dosage forms including capsules, tablets or granules. Concrete medicine can include aconitin and other pharmaceutically acceptable carriers, and the dosage of each component in the medicine can be adjusted according to the proportioning requirements of the components in the pharmaceutical preparation, and the effective content of aconitin can also be adjusted. It can be calculated according to the requirements of conventional dosage forms such as different dosage forms, 0.01375mg / kg to 0.055mg / kg body weight / d described in Example 1, this example only provides a reference value range here, and is not specifically limited. To further illustrate, the pharmaceutically acceptable carrier can use deodorants, fillers, disintegrants, coloring agents and thickeners, etc. Low-flavoring agents such a...
Embodiment 3
[0055] A tablet containing aconitin, each film-coated tablet containing 0.275 mg aconitin and 130 mg of auxiliary materials. Dissolve aconitin with food grade or pharmaceutical grade ethanol; mix excipients lactose 20mg, powdered sugar 25mg, microcrystalline cellulose 40mg and hydroxypropyl cellulose 10mg, then add aconitin ethanol and mix, then add Appropriate amount of purified water is mixed to make a soft material, which is then made into granules and then dried. Magnesium stearate and sodium carboxymethyl starch are added to the dried granules, pressed into tablets for a few minutes, and then coated with film.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com