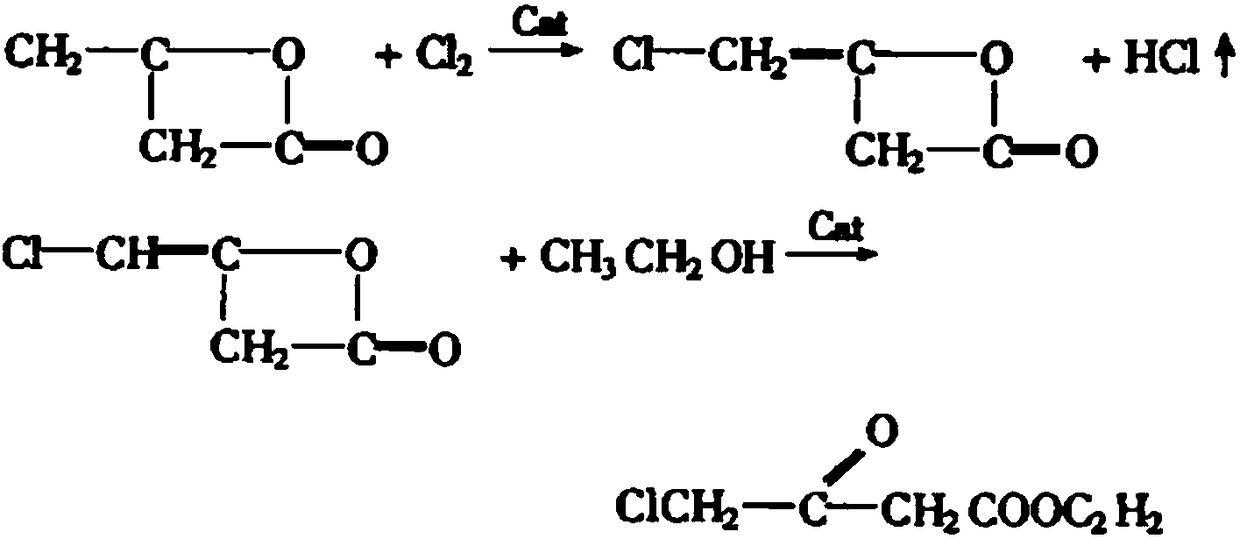
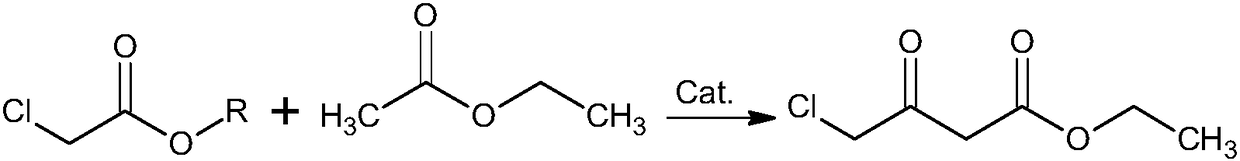
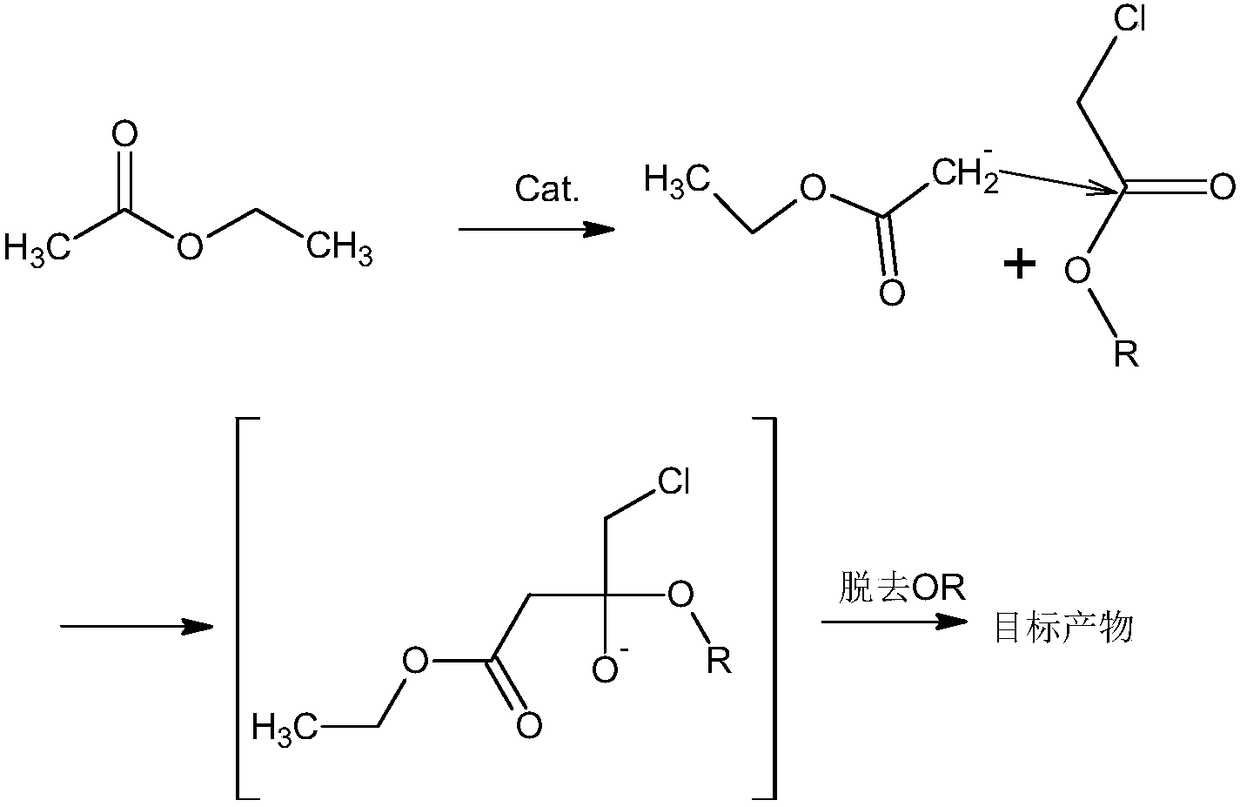
Method for synthesizing oxiracetam intermediate 4-ethyl chloroacetoacetate
A technology for the synthesis of ethyl chloroacetoacetate, which is applied to the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds, and can solve problems such as low product yield, decomposition loss of target products, and difficulties in separation and purification. Achieve the effect of high product purity, increase the probability of "collision" and low process cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The synthetic method of ethyl 4-chloroacetoacetate, comprises the following steps:
[0031] 1) Mix the ethyl acetate, the catalyst and DMSO evenly, pass in argon, control the pressure to 4.8 atmospheres, control the temperature to 128°C, add the solution of ethyl chloroacetate and ethanol dropwise, and control the addition time to 21min; After finishing, raise the reaction temperature to 152° C., raise the pressure to 9 atmospheres, and then continue to react for 22 hours to complete the reaction;
[0032] The preparation method of the catalyst used is as follows: the molecular sieve of type 5A is soaked in 20% potassium carbonate aqueous solution for 50 hours, dried in the air, and then activated at 450 DEG C to obtain.
[0033] The consumption ratio of ethyl acetate and catalyzer is 1g:0.31g, the consumption ratio of ethyl acetate and DMSO is 1g:6.8ml, the consumption ratio of ethyl acetate and ethyl chloroacetate is 1mol:0.97mol, ethyl chloroacetate and The usage ra...
Embodiment 2
[0036] The synthetic method of ethyl 4-chloroacetoacetate, comprises the following steps:
[0037] 1) Mix ethyl acetate, catalyst and DMF, feed nitrogen, control the pressure to 3 atmospheres, control the temperature to 110°C, add the solution of methyl chloroacetate and methanol dropwise, and control the addition time to 15min; , raising the reaction temperature to 145°C, raising the pressure to 8 atmospheres, and then continuing to react for 15 hours to complete the reaction;
[0038] The preparation method of the catalyst is as follows: immerse the 4A type molecular sieve in 15% potassium carbonate or sodium carbonate aqueous solution for 2 days, then dry it, and then activate it at 350°C.
[0039] The consumption ratio of ethyl acetate and catalyzer is 1g:0.25g, and the consumption ratio of described ethyl acetate and DMF is 1g:5.6ml, and the consumption ratio of described ethyl acetate and methyl chloroacetate is 1mol:0.95mol, so The consumption ratio of methyl chloroace...
Embodiment 3
[0042] The synthetic method of ethyl 4-chloroacetoacetate, comprises the following steps:
[0043] 1) Mix the ethyl acetate, the catalyst and DMSO evenly, pass in argon, control the pressure to 5 atmospheres, control the temperature to 135°C, add the solution of ethyl chloroacetate and ethanol dropwise, and control the addition time to 25min; After finishing, raise the reaction temperature to 160° C., raise the pressure to 10 atmospheres, and then continue to react for 25 hours to complete the reaction;
[0044] The preparation method of the catalyst is as follows: immerse the 5A type molecular sieve in 25% potassium carbonate or sodium carbonate aqueous solution for 3 days, then dry it, and then activate it at 470 DEG C to obtain it.
[0045] The consumption ratio of ethyl acetate and catalyzer is 1g:0.33g, and the consumption ratio of described ethyl acetate and DMSO is 1g:7.5ml, and the consumption ratio of described ethyl acetate and ethyl chloroacetate is 1mol:1mol, and d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com