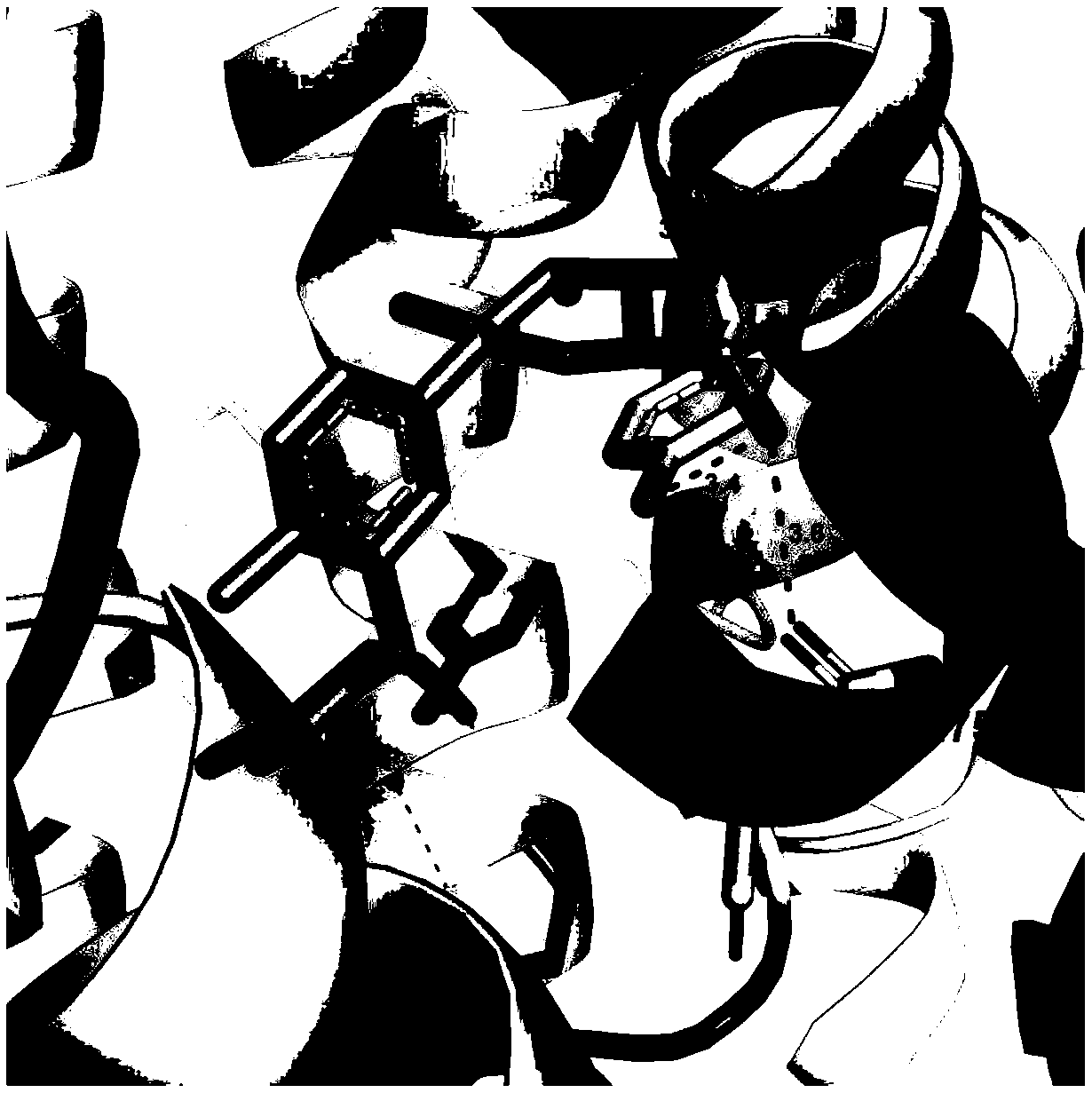Sodium-glucose cotransporter 2 (SGLT2) inhibitor and application thereof
A co-transporter and inhibitor technology, applied in the field of medicine, can solve problems such as affecting the effect of diabetes treatment, and achieve the effects of inhibiting glucose reabsorption, lowering blood sugar levels, and improving insulin resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
[0017] Example 1 Interaction between trelobatin and SGLT2 protein
[0018] Use the molecular docking simulation software Autodock and SGLT2 protein structure data to set the docking conditions with trilobatin, including: assuming that trilobatin interacts with SGLT2, on the one hand, the highly hydrophilic glycosyl part and the amino acid residues facing outward from the pocket On the other hand, trilobatin needs to have π-π interaction with the deeper hydrophobic part of the pocket. Special attention should be paid to the fact that the ASn75 and Tyr290 sites of SGLT2 are the key sites for its activity. Therefore, the key to determine whether trilobatin is an SGLT2 inhibitor is to see whether there is an interaction between trilobatin and these two sites.
[0019] The results of molecular docking confirmed that trilobatin interacts with SGLT2, and has hydrogen bonds with both Glu88 and Tyr263 sites of SGLT2. The specific docking results are as follows figure 1 shown.
Embodiment 2 3
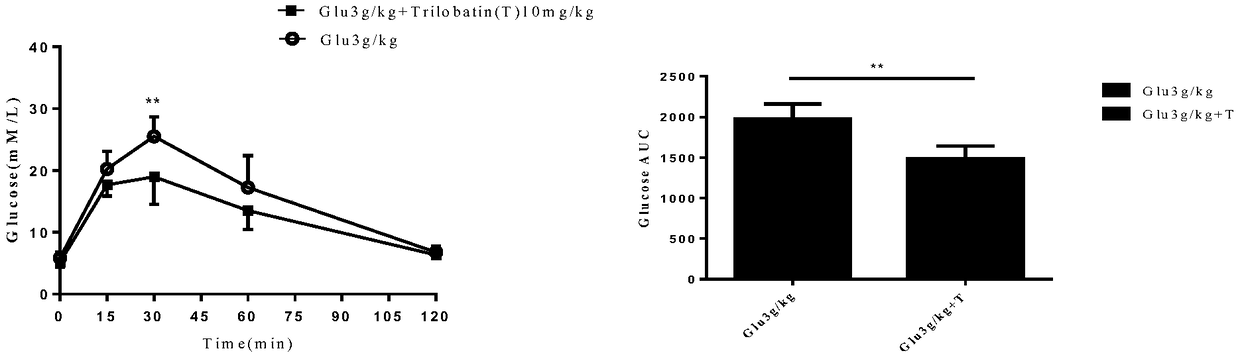
[0020] Embodiment 2 The influence of trilobatin on glucose reabsorption in experimental animals
[0021] The specific experimental steps are as follows:
[0022] (1) Select 6 10-week-old C57BL6 mice and divide them into two groups with 3 mice in each group;
[0023] (2) One group was administered with 2g / kg of glucose, and the other group was simultaneously administered with 2g / kg of glucose and 10mg / kg of trilobatin;
[0024] (2) Take blood from the tail vein at 0, 15, 30, 60 and 120 minutes respectively, and measure the blood sugar content with a Johnson & Johnson blood glucose meter.
[0025] Experimental results such as figure 2 shown, see figure 2 , Compared with the control group, the blood glucose levels of the experimental animals given trilobatin were significantly lower, indicating that trilobatin inhibits the absorption of glucose.
Embodiment 3 3
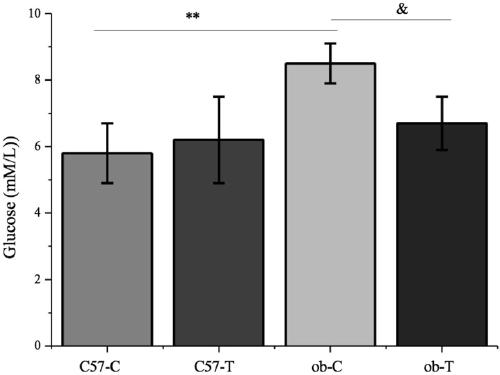
[0026] Example 3 Effect of trilobatin on blood sugar and glucose tolerance in insulin-resistant diabetic mice
[0027] The specific experimental steps are as follows:
[0028] (1) Select 12 10-12-week-old ob / ob mice and divide them into two groups. One group is the control group, and the other group is given 10 mg / kg trilobatin by intragastric administration every day for 4 consecutive weeks. C57BL6 mice of the same age were used as controls.
[0029] (2) After 4 weeks, the mice were starved for 12 hours, and the fasting blood glucose levels of the mice in each group were measured respectively;
[0030] (3) Simultaneously, by intragastric administration of glucose at 2 g / kg, blood was taken from the tail vein at 0, 15, 30, 60 and 120 minutes respectively, and the content of blood sugar was measured with a Johnson & Johnson blood glucose meter, and after intragastric administration of trilobatin, each Changes in glucose tolerance of group animals, to clarify whether trelobati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com