Patents
Literature
36 results about "Translocator protein 2" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
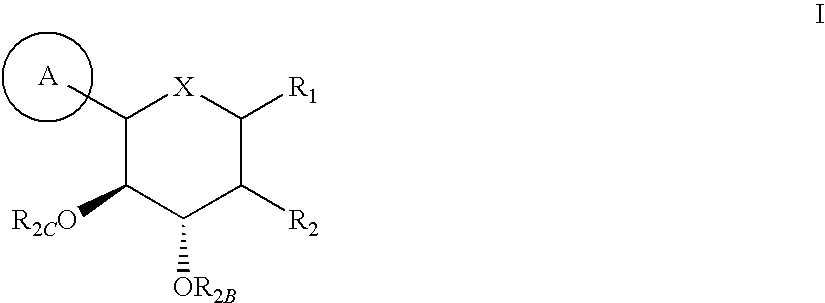
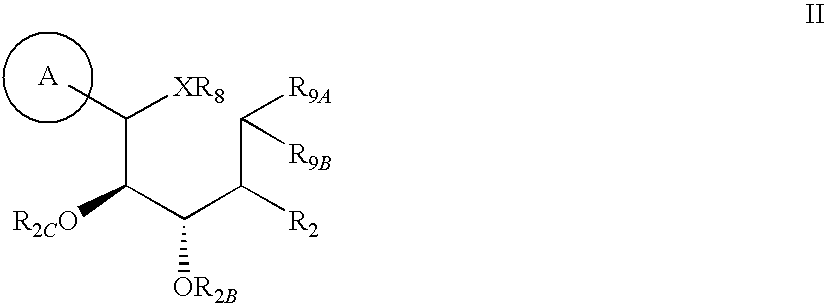
Substituted 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-ol compounds and methods relating thereto
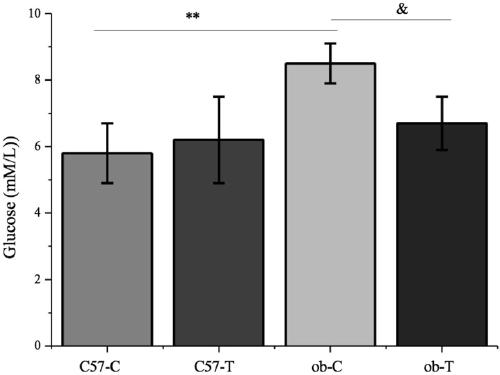
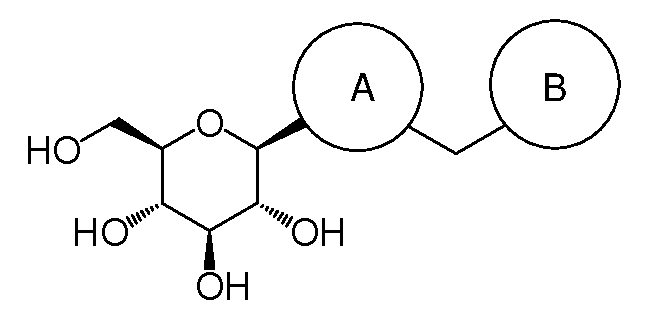
Substituted 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-ol compounds are disclosed that are inhibitors of the vesicular monoamine transporter 2 (VMAT2). The compounds of this invention have the structure:wherein R1 is as defined herein, including stereoisomers and pharmaceutically acceptable salts and solvates thereof. Also disclosed are compositions containing a compound of this invention in combination with a pharmaceutically acceptable carrier, as well as methods relating to the use in a subject in need thereof.
Owner:NEUROCRINE BIOSCI INC
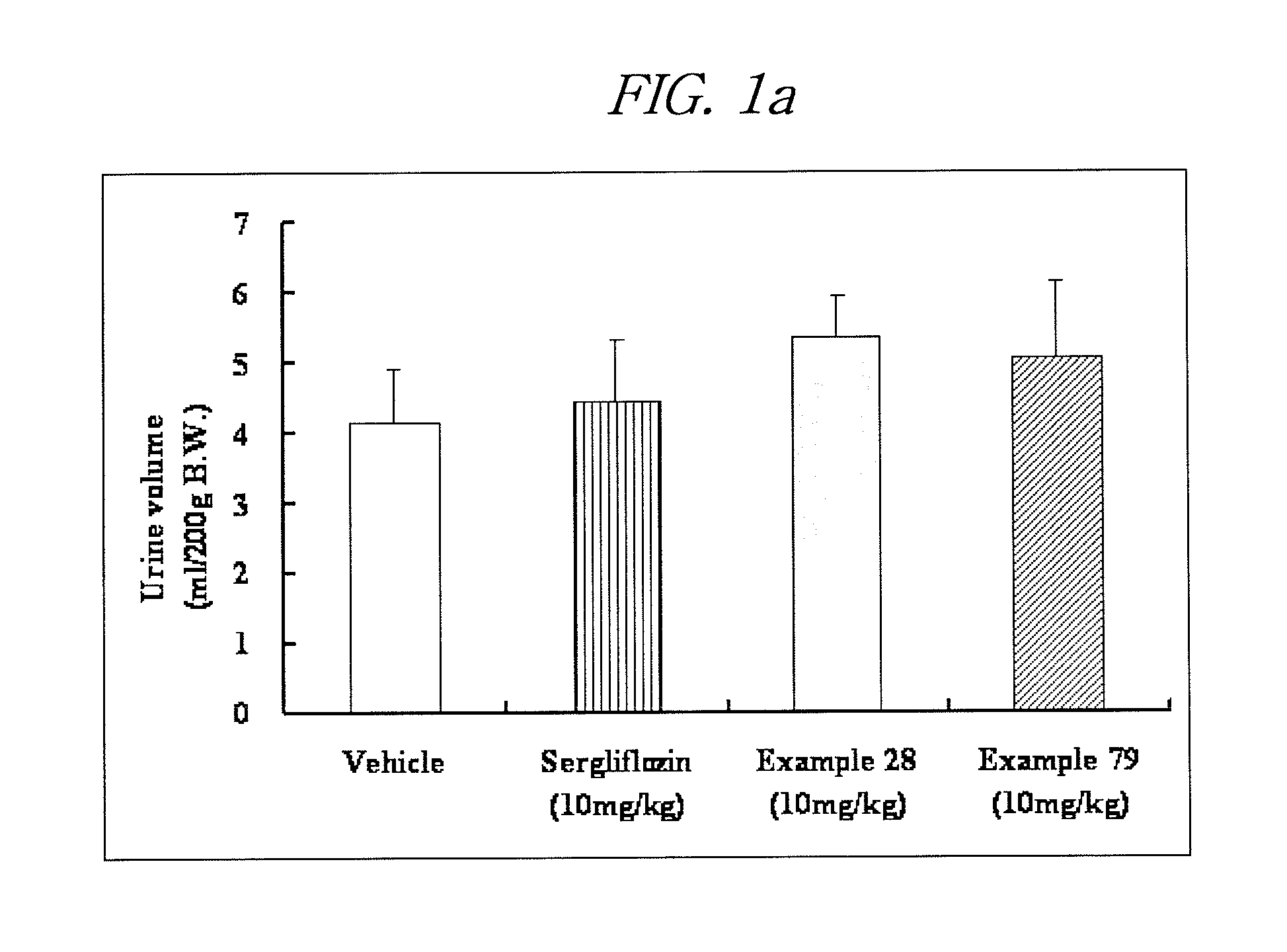
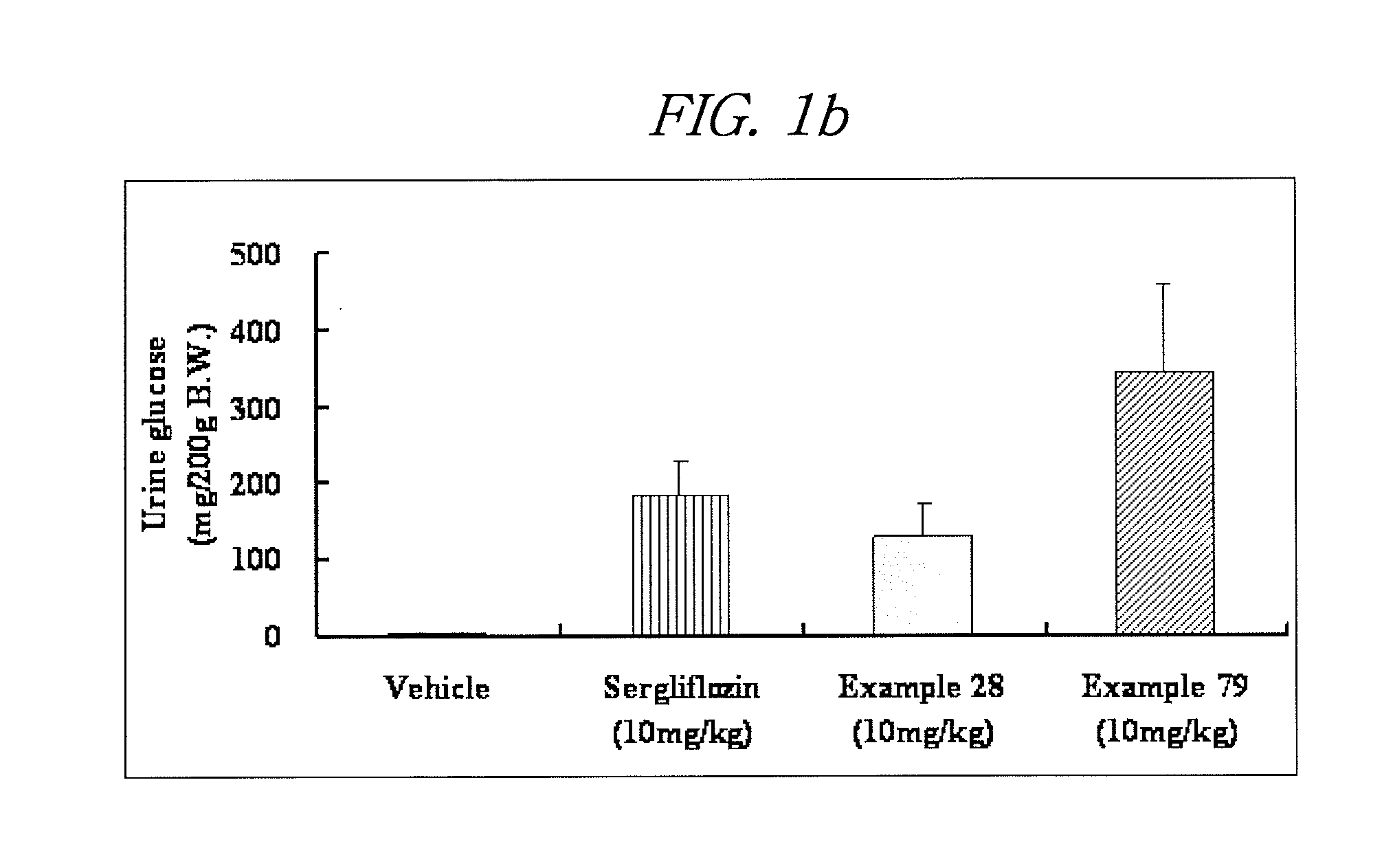
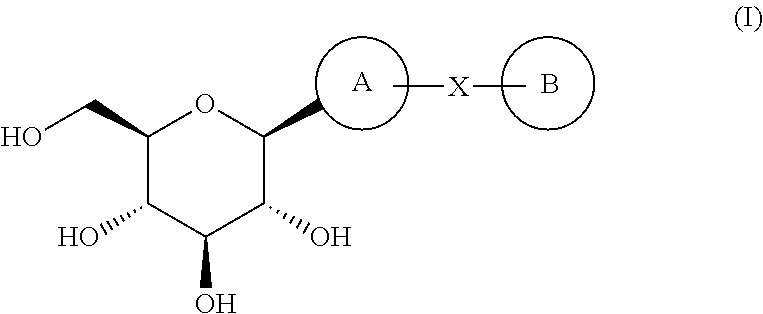
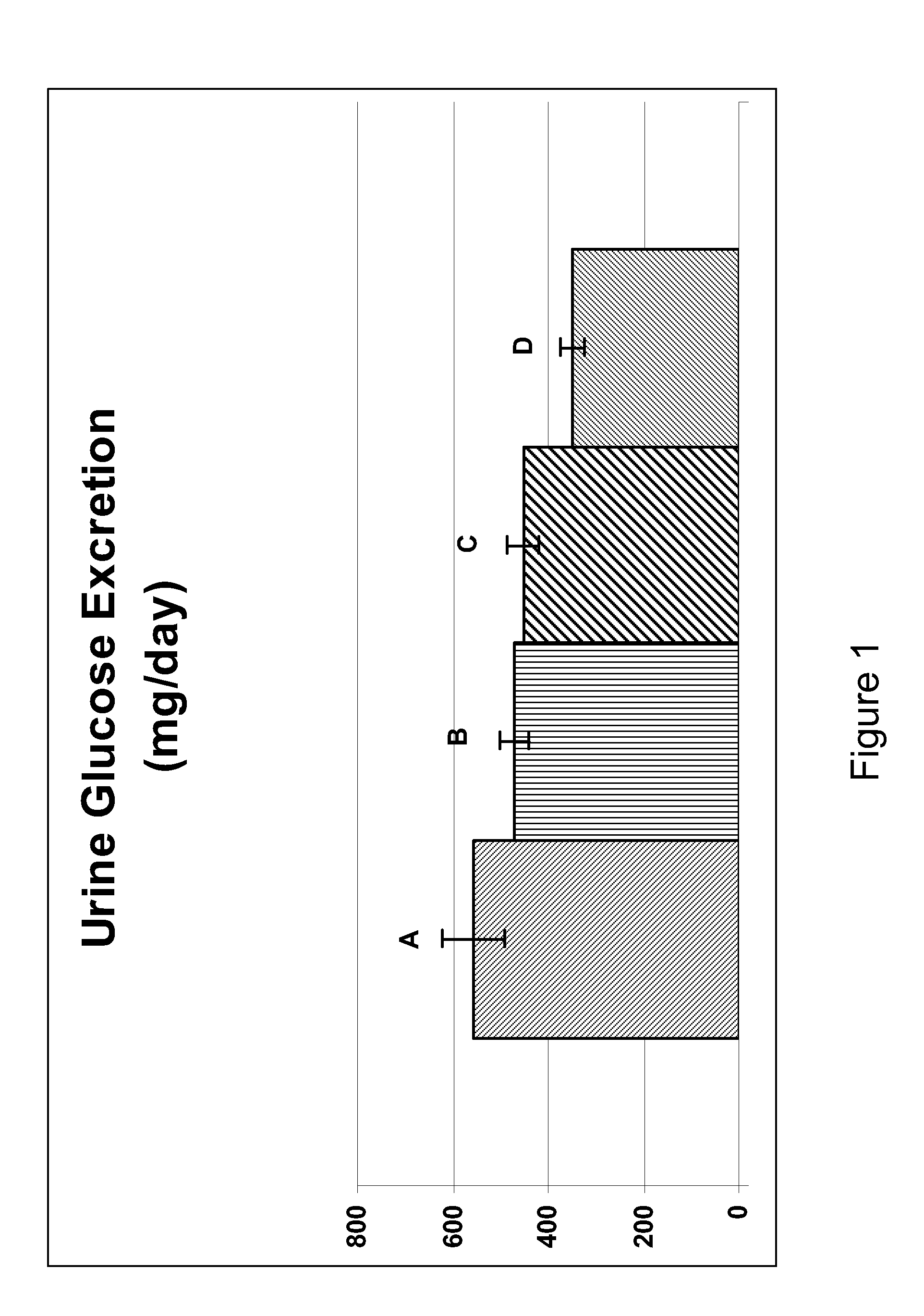
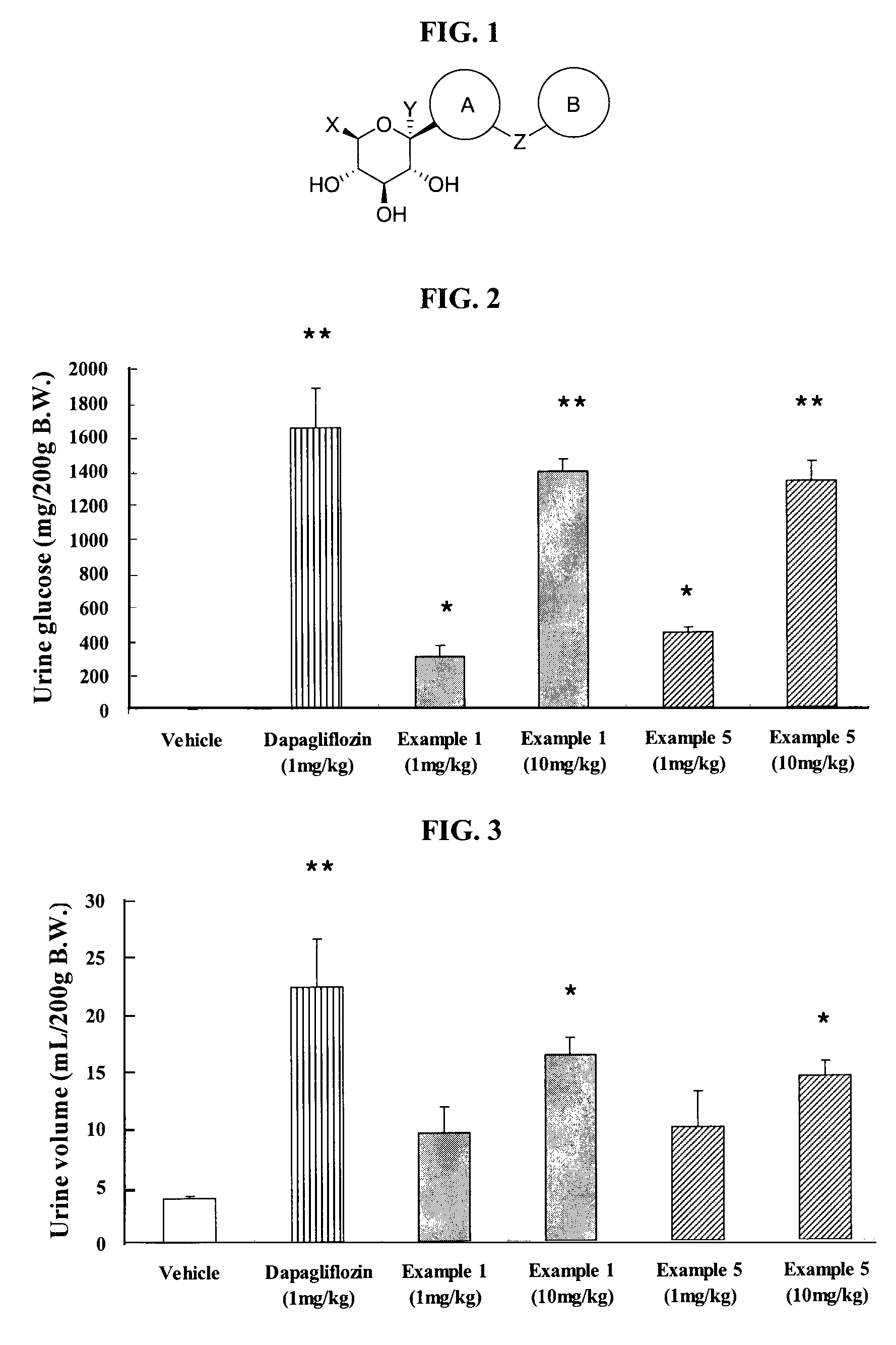
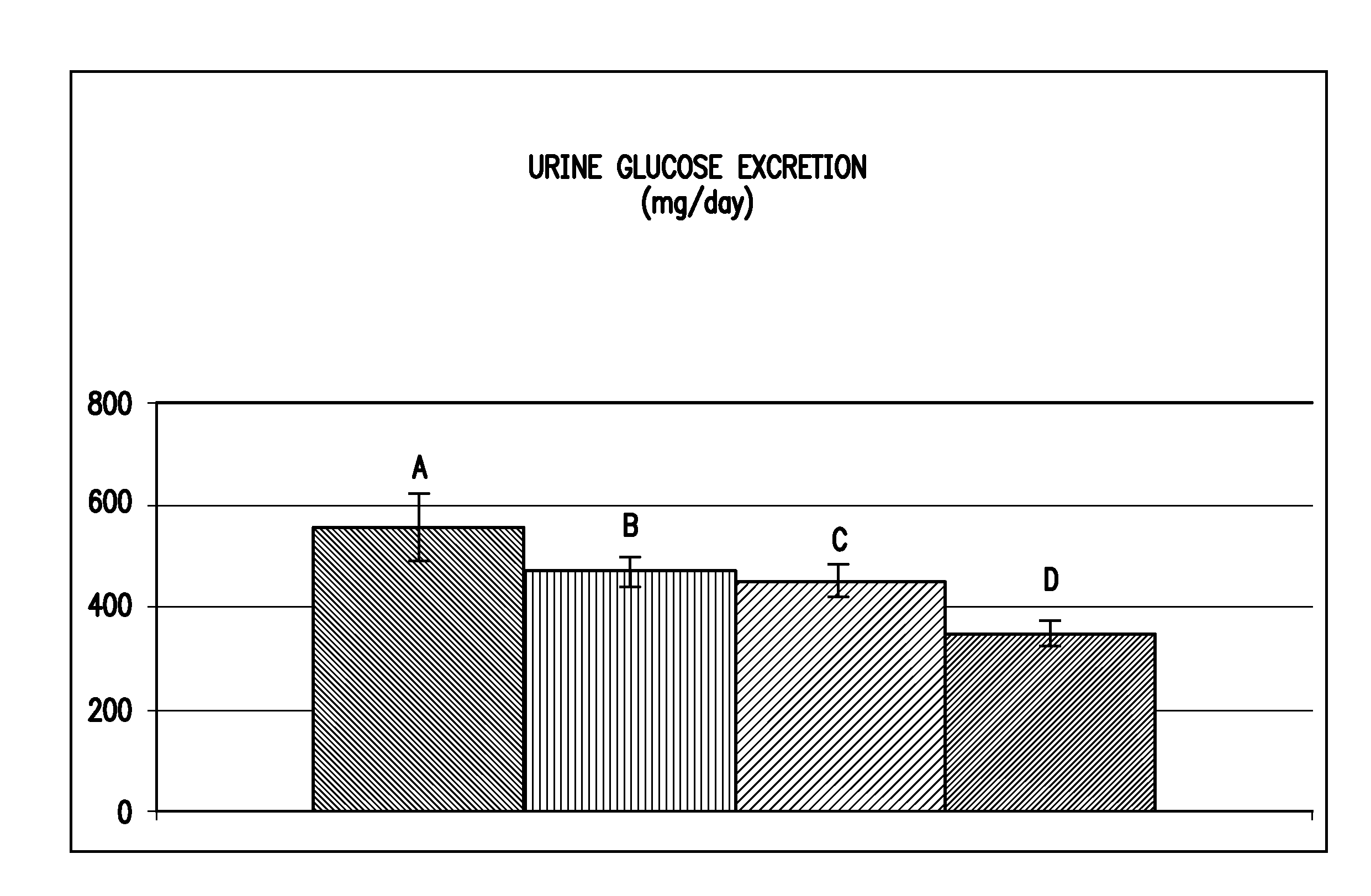
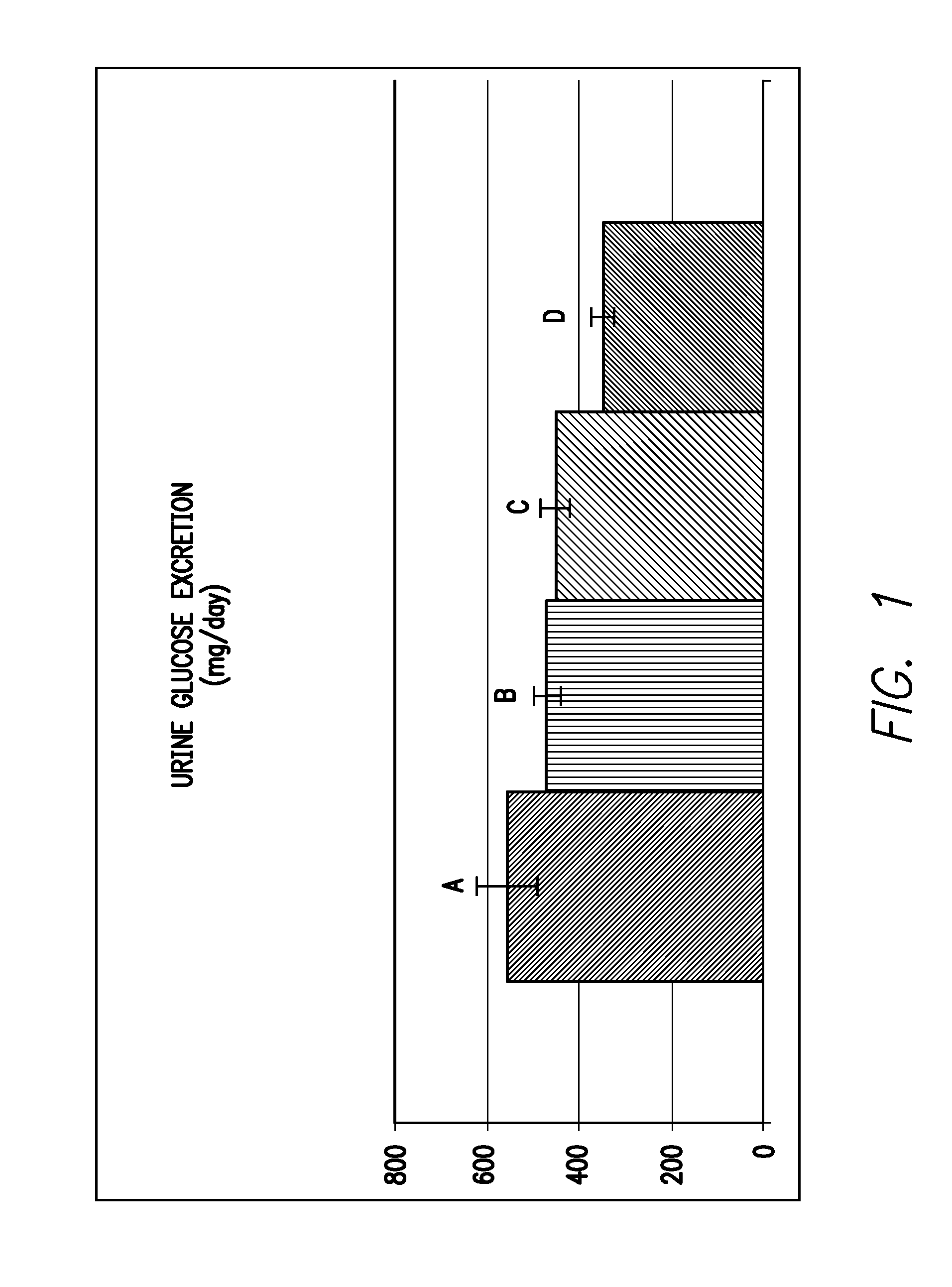
Inhibitors of sodium glucose co-transporter 2 and methods of their use
Compounds and pharmaceutical compositions comprising them are disclosed that may be useful for the treatment of diseases and disorders such as diabetes and obesity.
Owner:LEXICON PHARM INC
Thiazole derivatives as SGLT2 inhibitors and pharmaceutical composition comprising same
ActiveUS8586550B2Prevention and/or treatment of metabolic disordersReduce usageBiocideSaccharide with heterocyclic radicalsSodium dependentSGLT2 Inhibitor
The present invention relates to a novel compound with thiazole ring having an inhibitory activity against sodium-dependent glucose cotransporter 2 (SGLT2) being present in the intestine and kidney, and a pharmaceutical composition comprising the same as an active ingredient, which is useful for preventing or treating metabolic disorders, particularly diabetes.
Owner:THE GREEN CROSS CORP
Inhibitors of sodium glucose co-transporter 2 and methods of their use
ActiveCN101343296AInhibitory activityOrganic active ingredientsSugar derivativesDiseaseDiabetes mellitus
Compounds and pharmaceutical compositions comprising them are disclosed that may be useful for the treatment of diseases and disorders such as diabetes and obesity.
Owner:LEXICON PHARM INC
Inhibitors of Sodium Glucose Co-Transporter 2 and Methods of Their Use
Compounds and pharmaceutical compositions comprising them are disclosed that may be useful for the treatment of diseases and disorders such as diabetes and obesity.
Owner:LEXICON PHARM INC
Thiazole Derivatives as SGLT2 Inhibitors and Pharmaceutical Composition Comprising Same
ActiveUS20130090298A1Reduce usageUseful in treatmentBiocideSaccharide with heterocyclic radicalsIntestinal structureBULK ACTIVE INGREDIENT
The present invention relates to a novel compound with thiazole ring having an inhibitory activity against sodium-dependent glucose cotransporter 2 (SGLT2) being present in the intestine and kidney, and a pharmaceutical composition comprising the same as an active ingredient, which is useful for preventing or treating metabolic disorders, particularly diabetes.
Owner:THE GREEN CROSS CORP
Anti-system asc amino acid transporter 2 (ASCT2) antibody
An object of the present invention is to provide a monoclonal antibody which is useful for treating or diagnosing a disease relating to system ASC amino acid transporter 2 (hereinafter, referred to as “ASCT2”) or a method using the antibody. The present invention provides a monoclonal antibody which specifically recognizes a native three-dimensional structure of an extracellular region of ASCT2 and binds to the extracellular region, or an antibody fragment thereof; a hybridoma which produces the antibody; a DNA which encodes the antibody; a vector which contains the DNA; a transformant obtainable by introducing the vector; a process for producing an antibody or an antibody fragment thereof using the hybridoma or the transformant; and a therapeutic agent using the antibody or the antibody fragment thereof, and a diagnostic agent using the antibody or the antibody fragment thereof.
Owner:KYOWA HAKKO KIRIN CO LTD
Inhibitors of sodium glucose co-transporter 2 and methods of their use
ActiveUS7781577B2Saccharide with heterocyclic radicalsSaccharide with carbocyclic radicalsDiabetes mellitusD-Glucose
Compounds and pharmaceutical compositions comprising them are disclosed that may be useful for the treatment of diseases and disorders such as diabetes and obesity.
Owner:LEXICON PHARM INC
Anti system asc amino acid transporter 2 (ASCT2) antibody
An object of the present invention is to provide a monoclonal antibody which is useful for treating or diagnosing a disease relating to system ASC amino acid transporter 2 (ASCT2) or a method using the antibody. The present invention provides a monoclonal antibody which specifically recognizes a native three-dimensional structure of an extracellular region of system ASC amino acid transporter 2 (ASCT2) and binds to the extracellular region, or an antibody fragment thereof; a hybridoma which produces the antibody; a DNA which encodes the antibody; a vector which contains the DNA; a transformant obtainable by introducing the vector; a process for producing an antibody or an antibody fragment thereof using the hybridoma or the transformant; and a therapeutic agent using the antibody or the antibody fragment thereof, and a diagnostic agent using the antibody or the antibody fragment thereof.
Owner:KYOWA HAKKO KIRIN CO LTD
1-(beta-d-pyranoglucosyl)-4-methyl-3-[5-(4-fluorophenyl)-2-thienylmethyl]benzene and l-phenylalanine eutectic compound and preparation method thereof
InactiveCN103965267AImprove stabilityGood for medicineSaccharide with carbocyclic radicalsSugar derivativesBenzeneD-Glucose
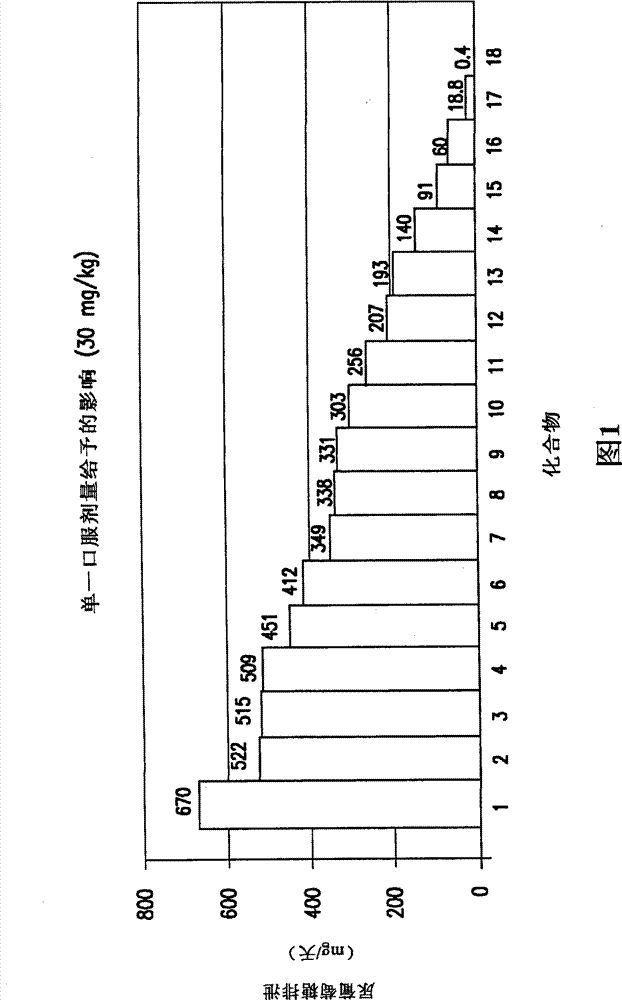
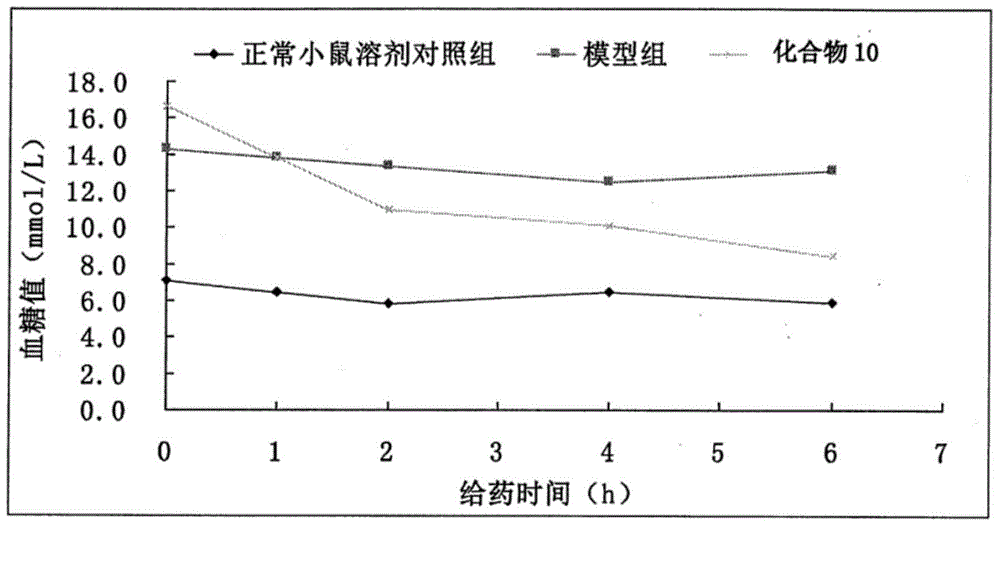
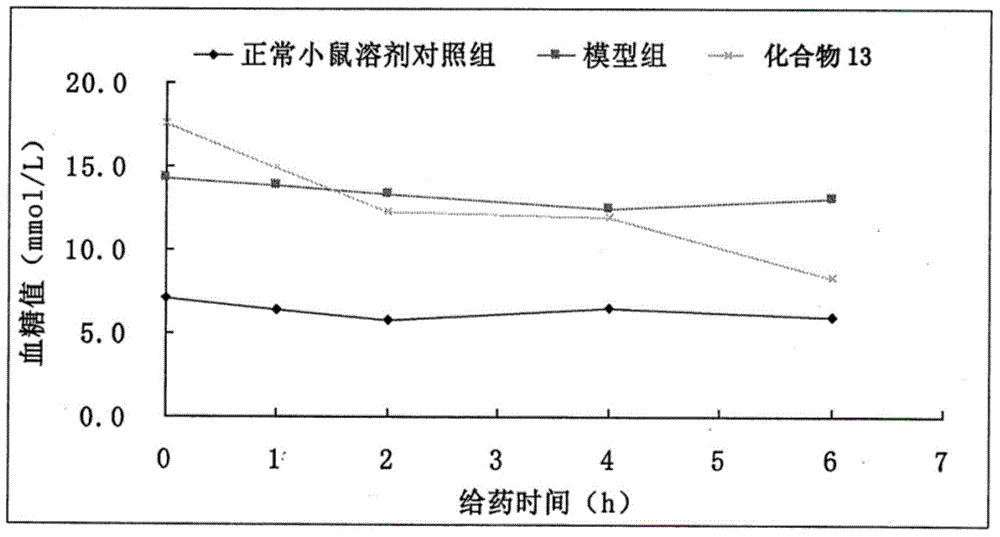
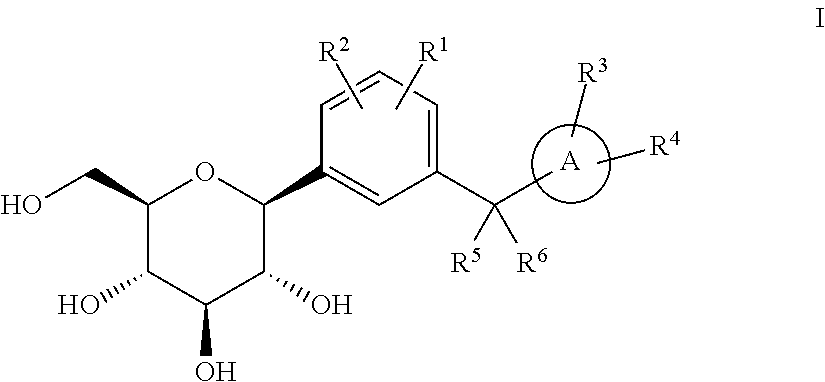
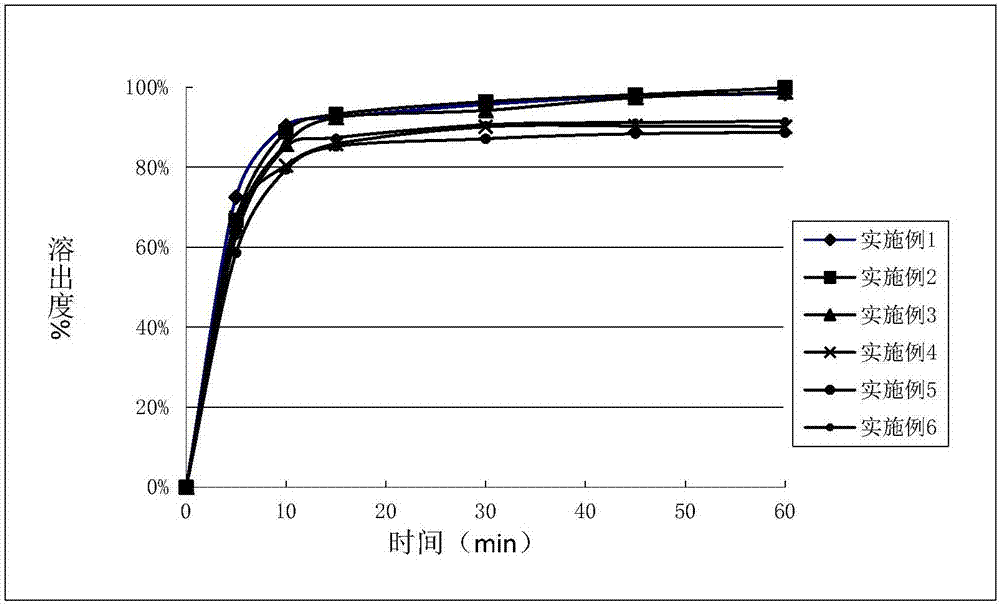
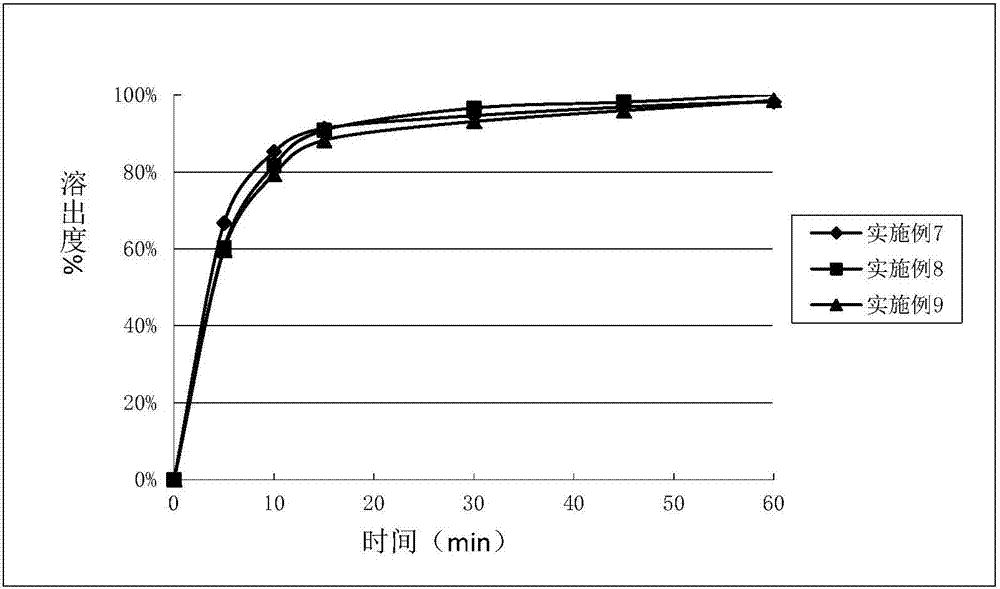
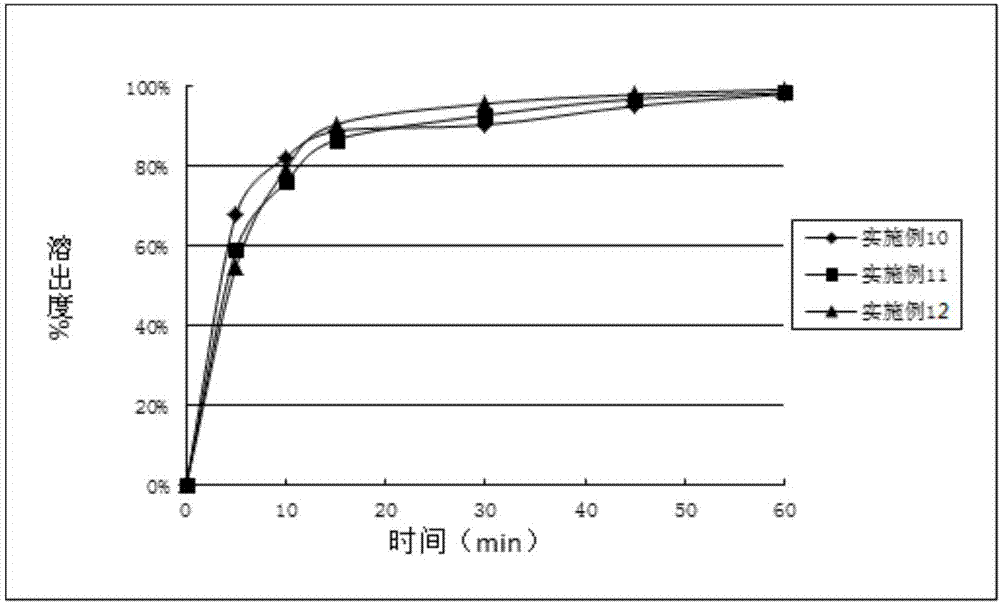
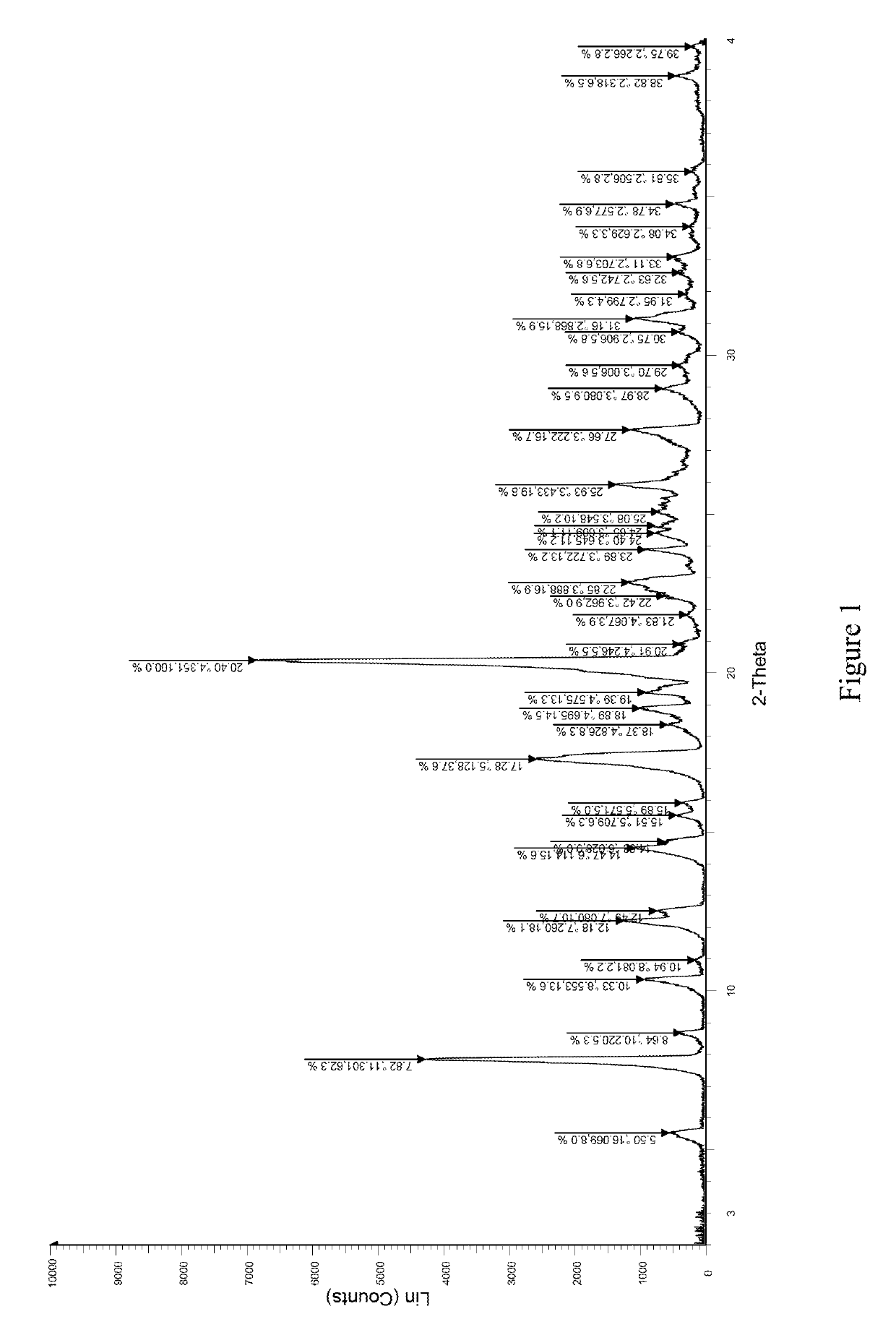
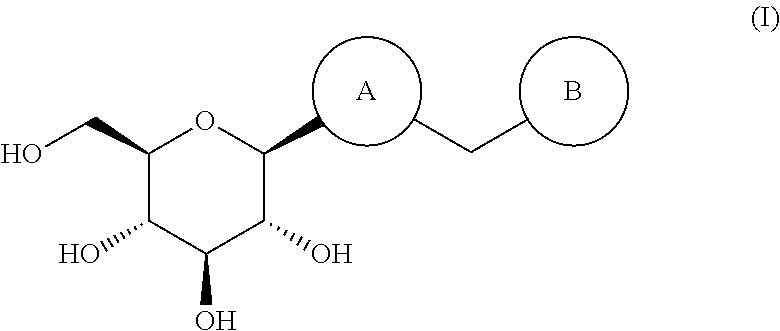
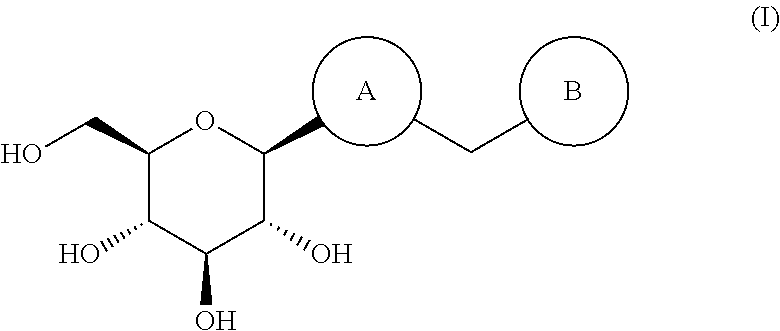
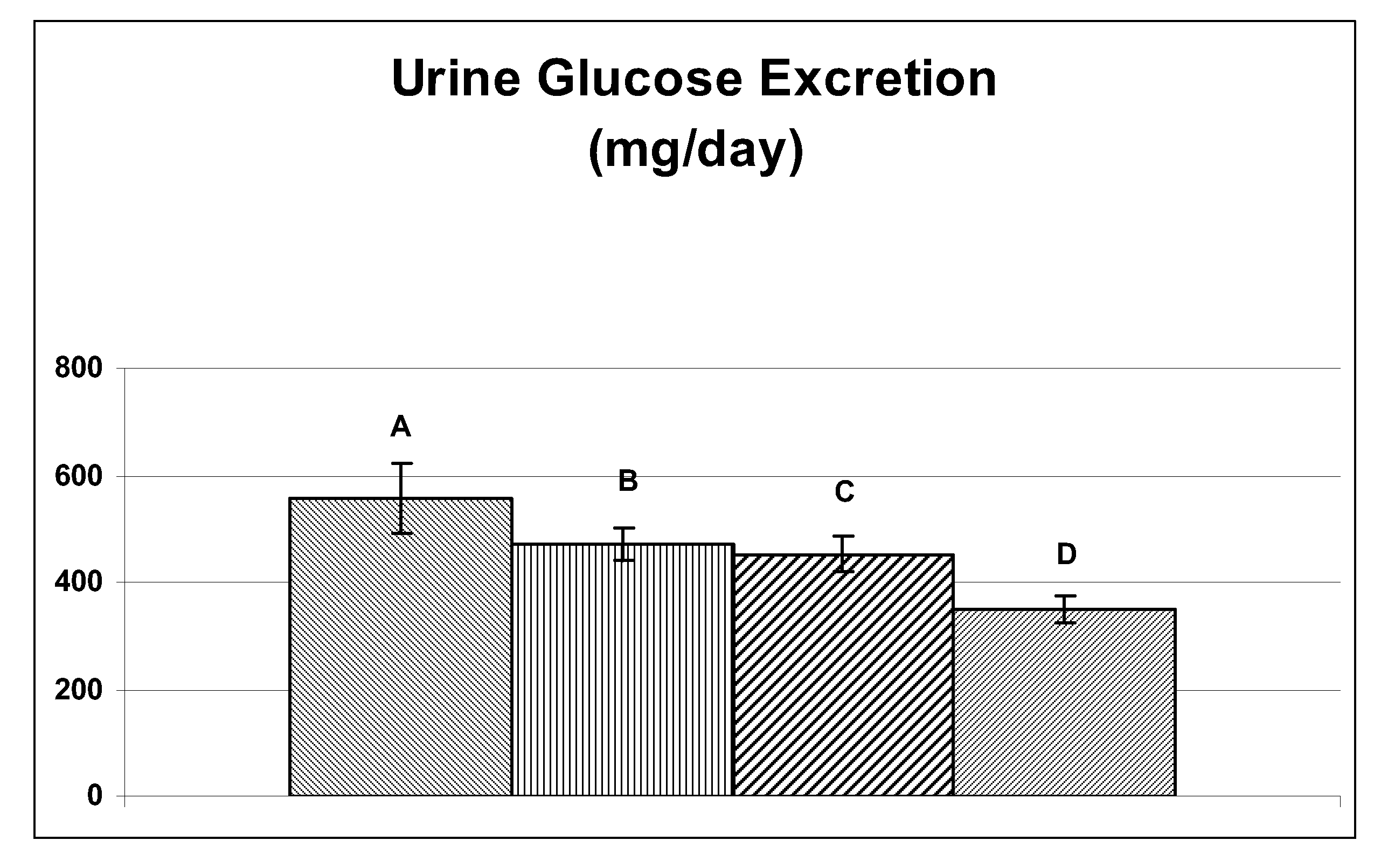
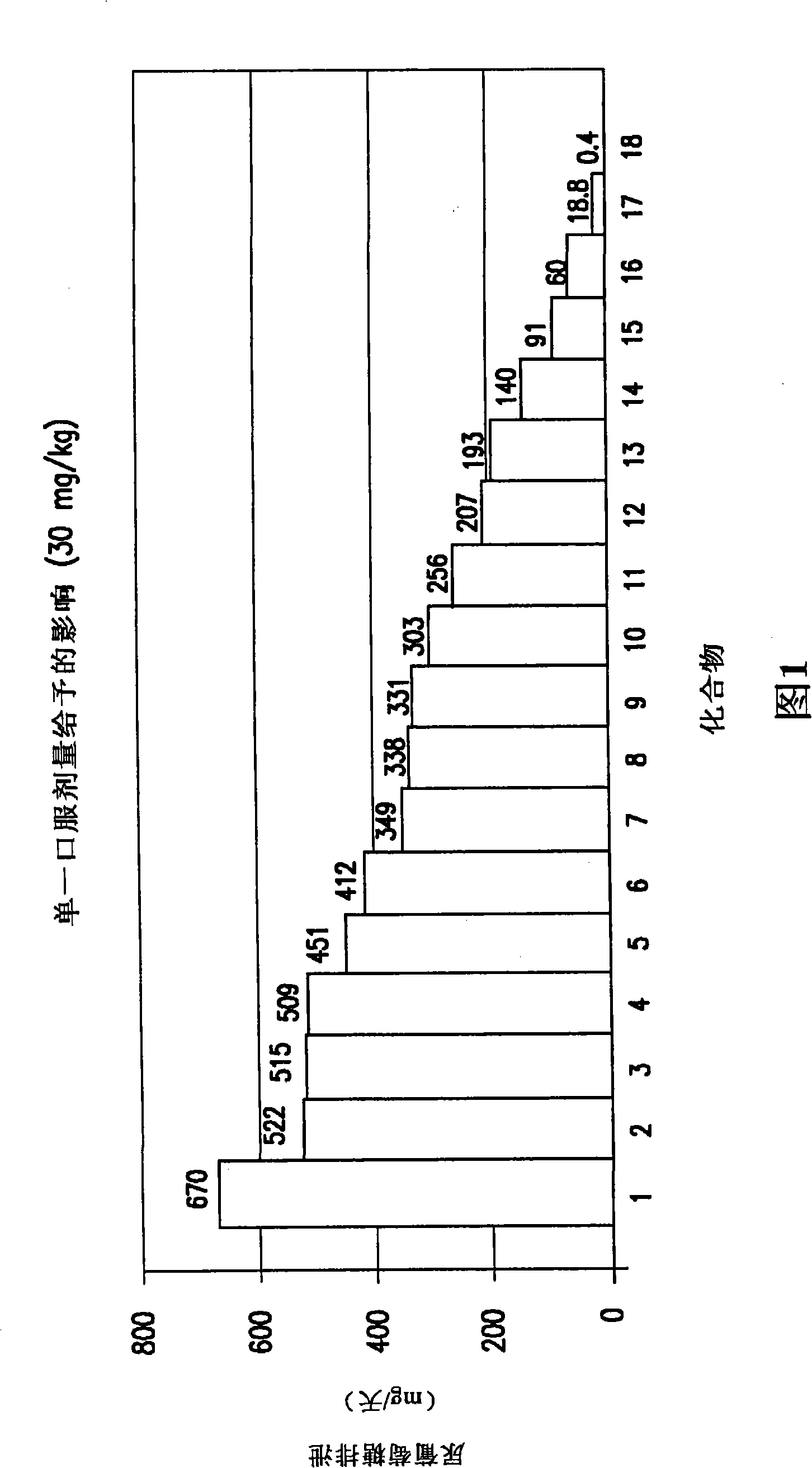
The invention discloses an eutectic compound of 1-(beta-D-pyranoglucosyl)-4-methyl-3-[5-(4-fluorophenyl)-2-thienylmethyl]benzene and L-phenylalanine and a preparation method thereof. Specifically speaking, the invention discloses the eutectic compound shown in a formula (Ia) and formed by L-phenylalanine and a sodium-glucose co-transporter 2 (SGLT-2) inhibitor shown in a formula (I), i.e., 1-(beta-D-pyranoglucosyl)-4-methyl-3-[5-(4-fluorophenyl)-2-thienylmethyl]benzene. The XRD characteristic peaks of the eutectic compound are shown in a figure 1. The preparation method comprises the following steps: mixing an aqueous solution of L-phenylalanine with an ethanol solution of the compound I; carrying out heating for dissolving of the two substances; then carrying out stirring, cooling and crystallization; and subjecting a filtrate to ethanol water recrystallization so as to obtain the eutectic compound. The formula (I) and the formula (Ia) are described in the specification.
Owner:JIANGSU HANSOH PHARMA CO LTD +1
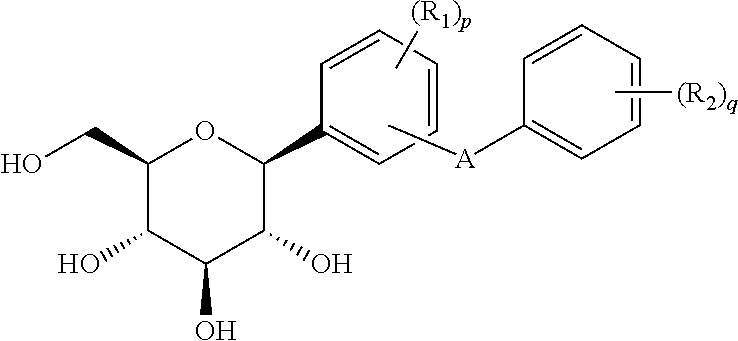
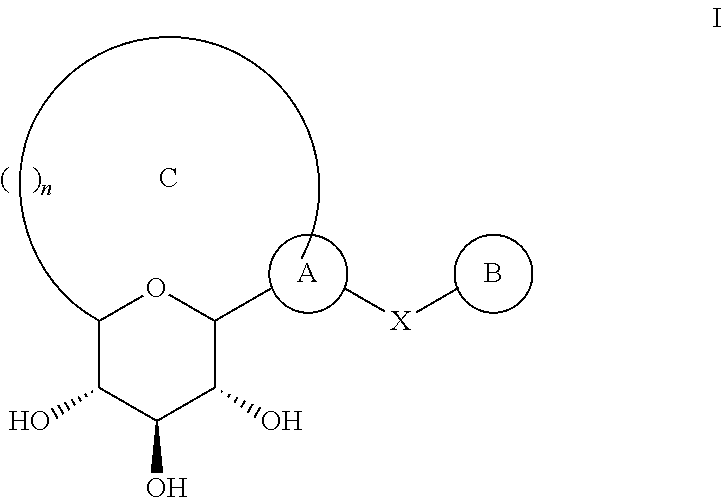
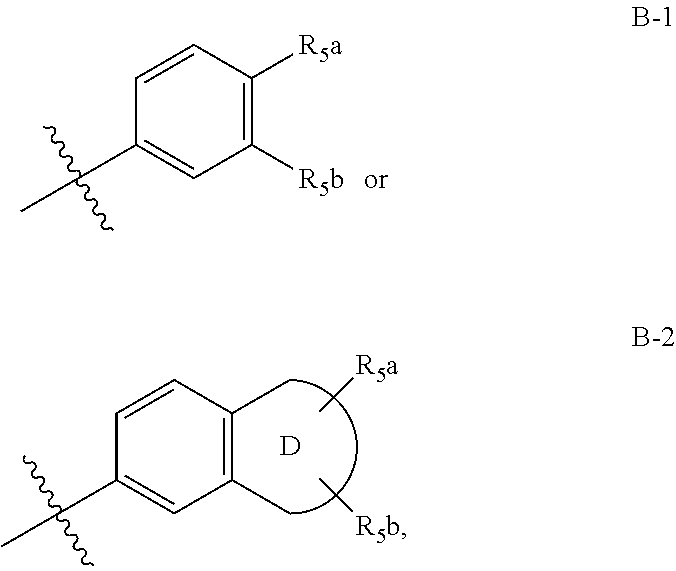
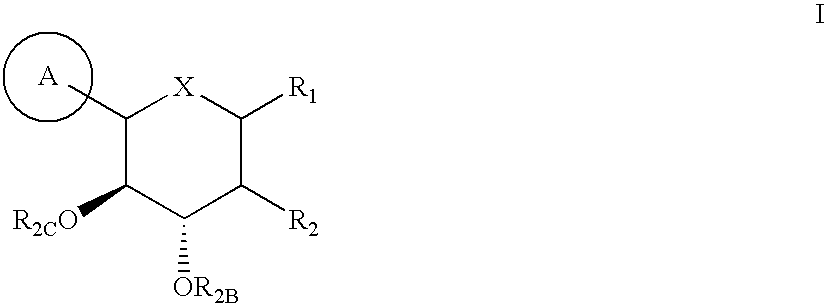
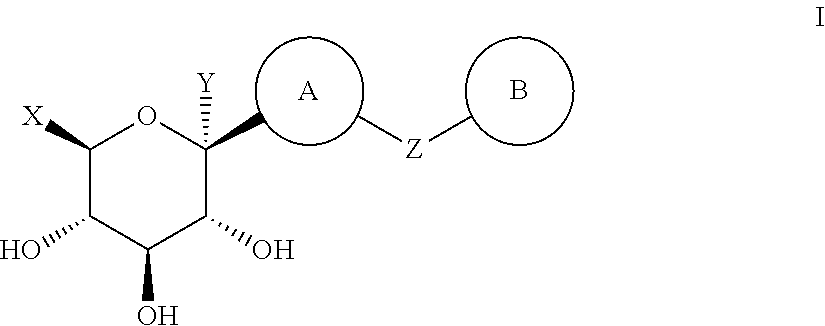
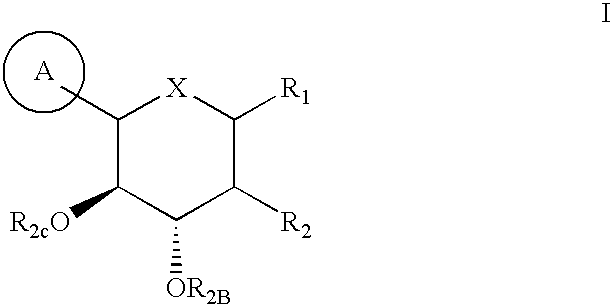
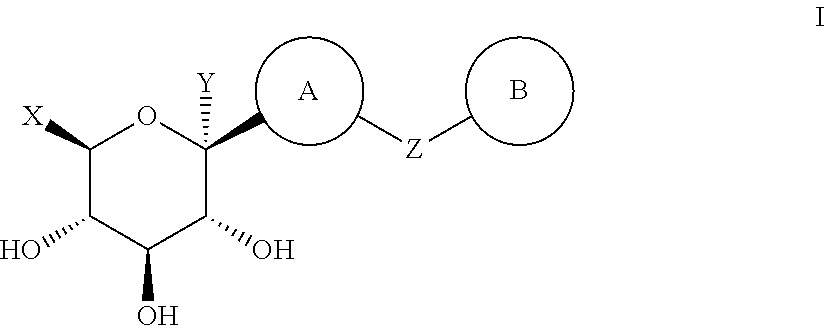
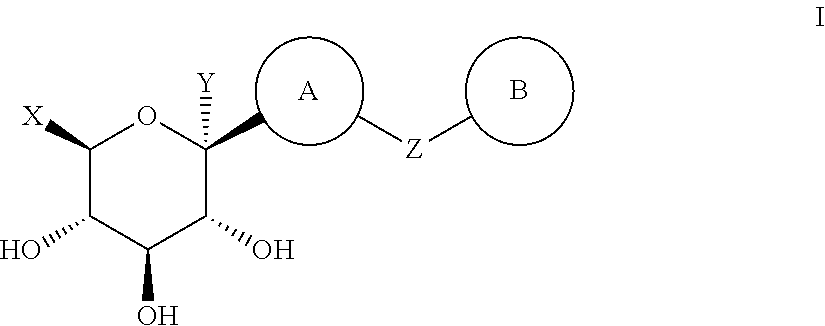
Method for dual inhibition of SGLT1 and SGLT2 using diphenylmethane derivatives
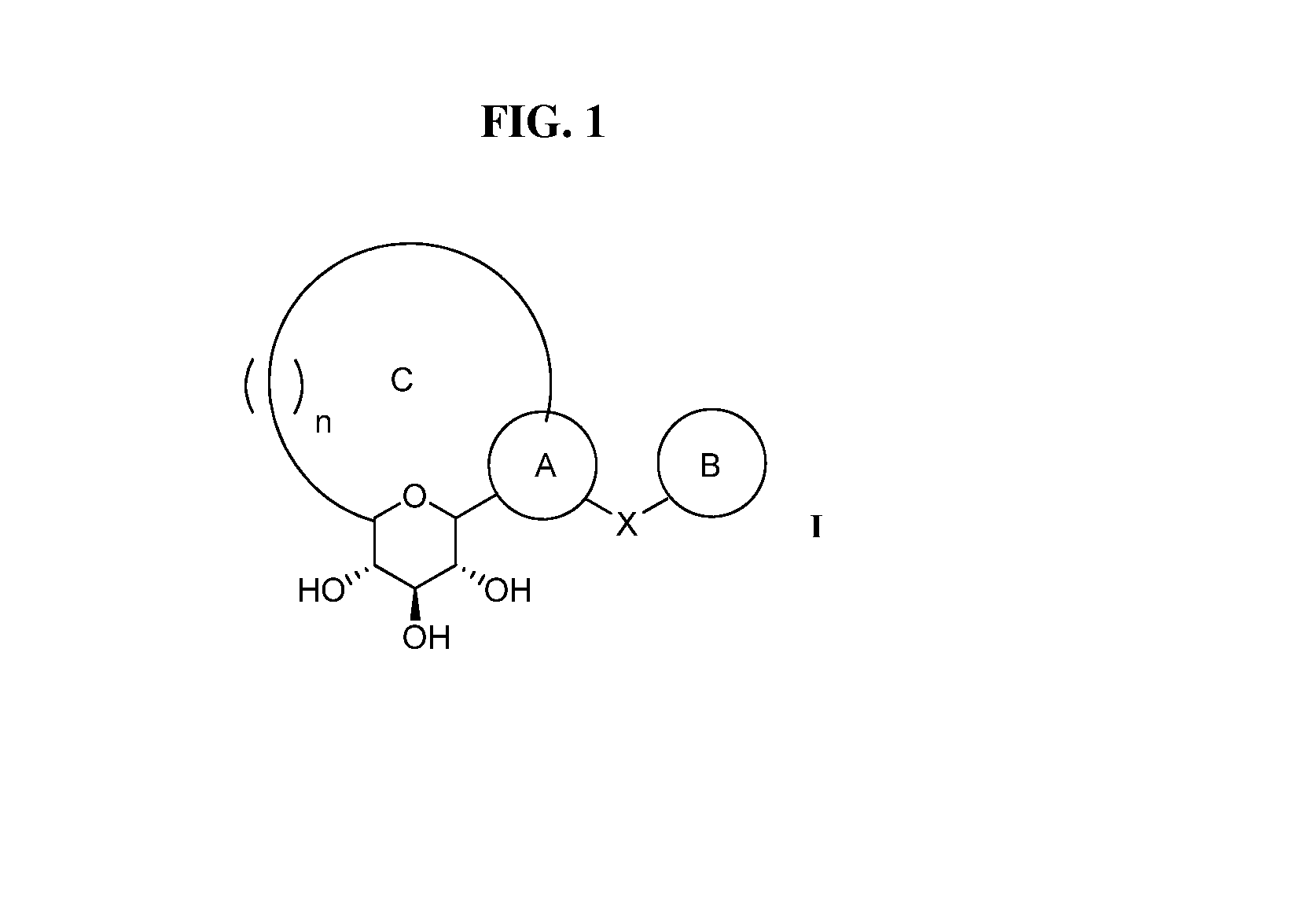
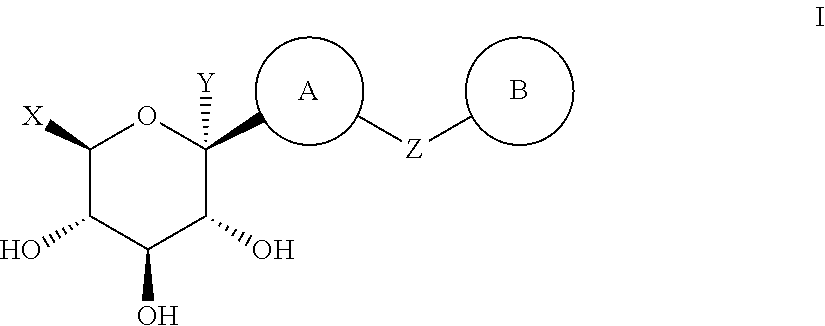
The present invention relates to a method for dual inhibition of sodium-dependent glucose cotransporter 1 (SGLT1) and sodium-dependent glucose cotransporter 2 (SGLT2) present in the intestine and kidney by using the compound of formula I or a pharmaceutically acceptable salt or a prodrug thereof:wherein ring A, ring B, X and Y have the same meanings as defined in the specification.
Owner:THE GREEN CROSS CORP
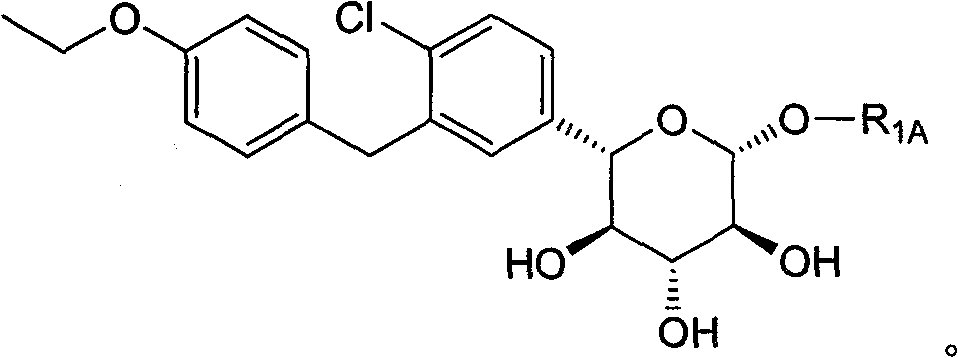
Preparation method suitable for industrial production of empagliflozin
InactiveCN109988161AHigh puritySimple purification methodOrganic chemistryGlucono delta-lactoneOrganic synthesis
The invention belongs to the technical field of organic synthesis route design and medicine and chemical engineering, particularly relates to a synthesis method of a sodium-glucose cotransporter 2(SGLT2) inhibitor, and more particularly relates to a preparation method of empagliflozin. The empagliflozin is synthesized by taking (3S)-3-[4-[(2-chloro-5-iodophenyl) methyl] phenoxy] tetrahydrofuran and glucono delta-lactone as initial raw materials through a series of substep reactions such as protection, addition, substitution, deprotection and reduction. In the synthesis steps disclosed by the invention, a staged target product does not need to be separated and purified after each step of reaction, and the target product is finally obtained by directly subjecting a high-purity reaction intermediate to subsequent steps. The preparation method is simple in process, simple and convenient to operate and good in industrial prospect.
Owner:XUZHOU WANBANG JINQIAO PHARMA +1
Glucoside derivatives and pharmaceutical compositions thereof
InactiveCN104817554AGood physical and chemical propertiesLow toxicityOrganic active ingredientsSenses disorderDiabetic retinopathyDisease
The invention provides glucoside derivatives and pharmaceutical compositions thereof. The invention discloses chemical compounds as shown in general formula (I), pharmaceutically-acceptable salts thereof, easily-hydrolytic lipid prodrug thereof or isomers thereof, and the pharmaceutical compositions containing the chemical compounds which is used as sodium-glucose cotransporter 2 inhibitor used for treating or suspending relative diseases like diabetes mellitus, diabetic retinopathy, diabetic neuropathy, diabetic nephropathy and insulin resistance.
Owner:JIANGSU ATOM BIOSCI & PHARMA CO LTD
Anti system ASC amino acid transporter 2 (ASCT2) antibody
ActiveUS8501180B2Useful in therapyBacteriaImmunoglobulins against cell receptors/antigens/surface-determinantsExtracellularDisease
An object of the present invention is to provide a monoclonal antibody which is useful for treating or diagnosing a disease relating to system ASC amino acid transporter 2 (ASCT2) or a method using the antibody. The present invention provides a monoclonal antibody which specifically recognizes a native three-dimensional structure of an extracellular region of system ASC amino acid transporter 2 (ASCT2) and binds to the extracellular region, or an antibody fragment thereof; a hybridoma which produces the antibody; a DNA which encodes the antibody; a vector which contains the DNA; a transformant obtainable by introducing the vector; a process for producing an antibody or an antibody fragment thereof using the hybridoma or the transformant; and a therapeutic agent using the antibody or the antibody fragment thereof, and a diagnostic agent using the antibody or the antibody fragment thereof.
Owner:KYOWA HAKKO KIRIN CO LTD
Benzimidazole derivatives and medical uses thereof
InactiveUS20080038242A1Lower uric acid levelsAvoid absorptionBiocidePeptide/protein ingredientsHyperuricemic nephropathyDisease
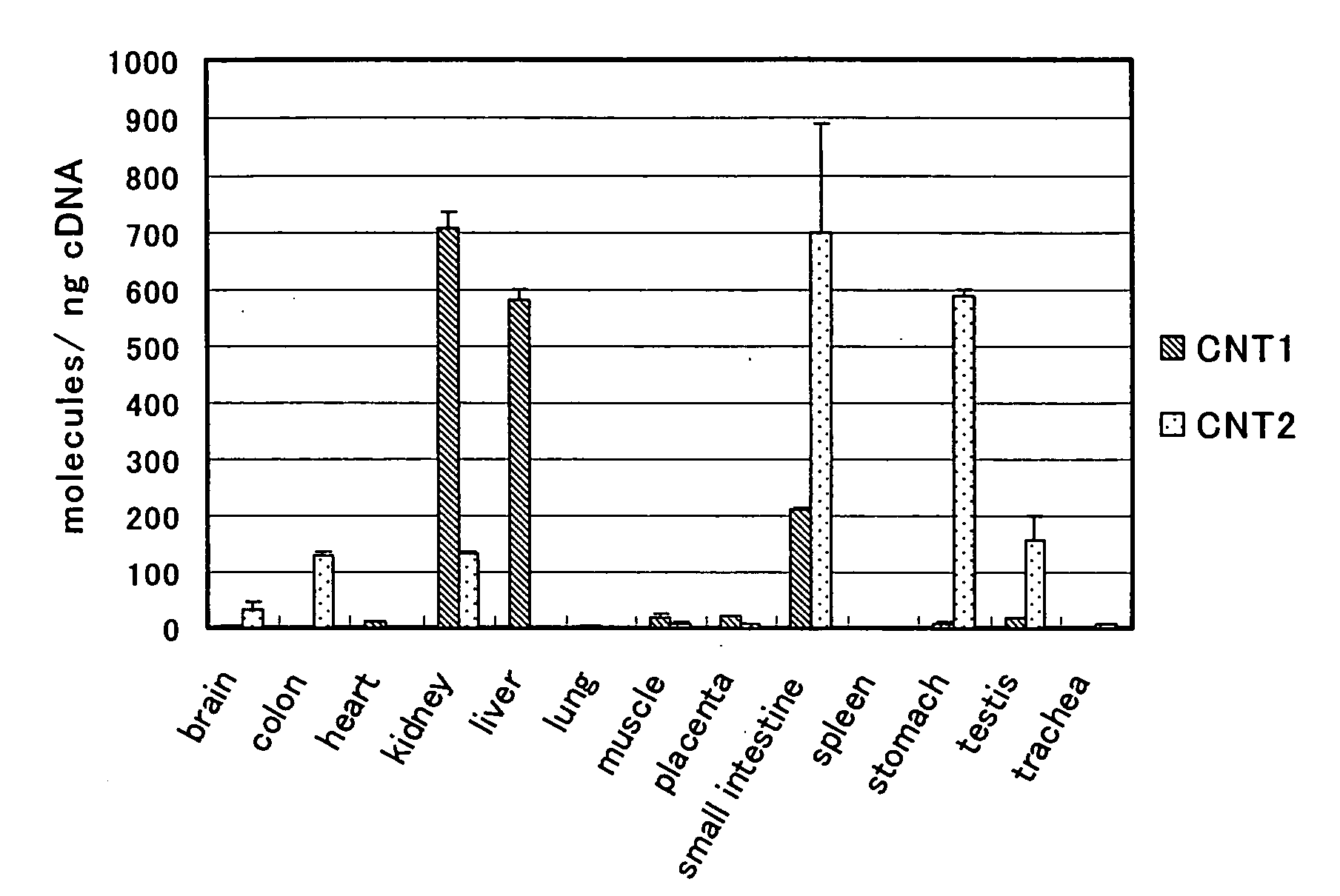
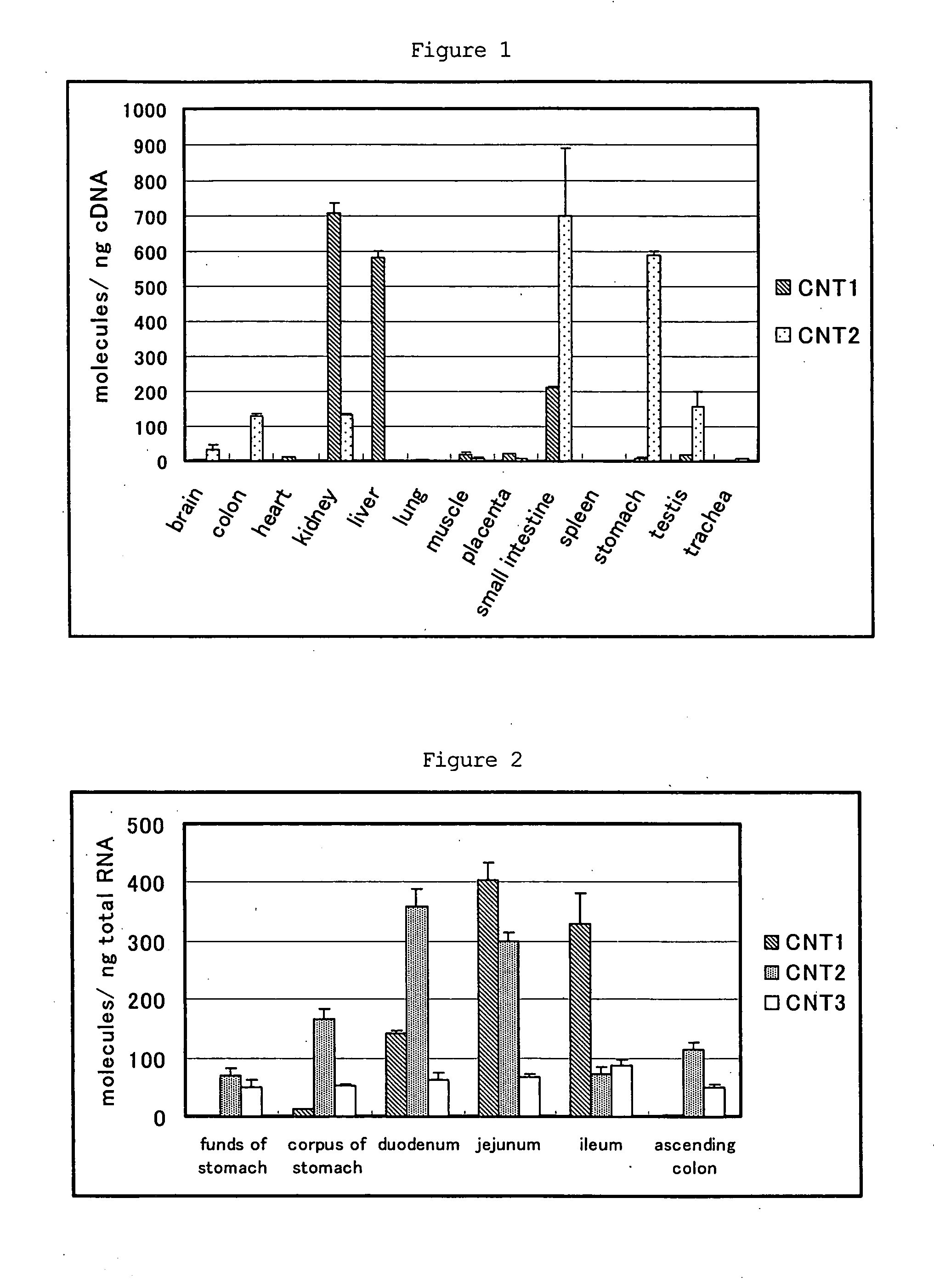
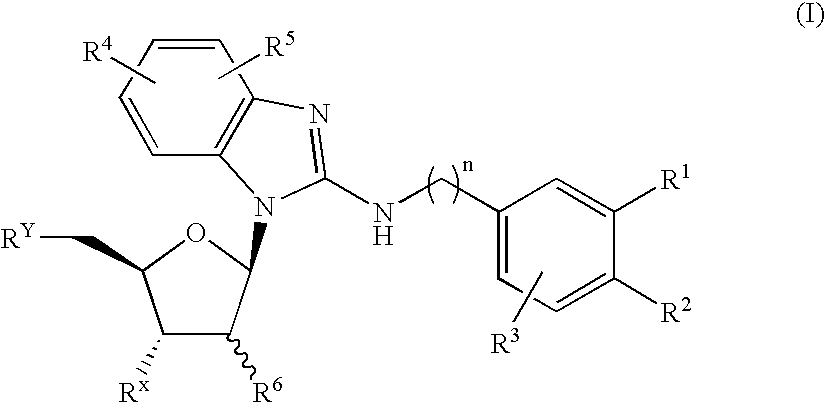
The present invention provides benzimidazole derivatives represented by the following formula (I) or pharmaceutically acceptable salts thereof, or prodrugs thereof, which exert an inhibitory activity on sodium-dependent nucleoside transporter 2 and are useful for a disease associated with an abnormality of plasma uric acid level. The compounds of the present invention are useful for the prevention or treatment of gout, hyperuricemia, urinary lithiasis, hyperuricemic nephropathy or the like. In the formula, n is 1 or 2; R1 and R2 are H, a halogen atom, cyano group, optionally substituted alkyl group, optionally substituted aryl group or the like; R3 is H, a halogen atom, optionally substituted alkyl group or the like; R4 and R5 are H, a halogen atom, OH or the like; and R6 and RX are H or OH: RY is F or OH.
Owner:KISSEI PHARMA
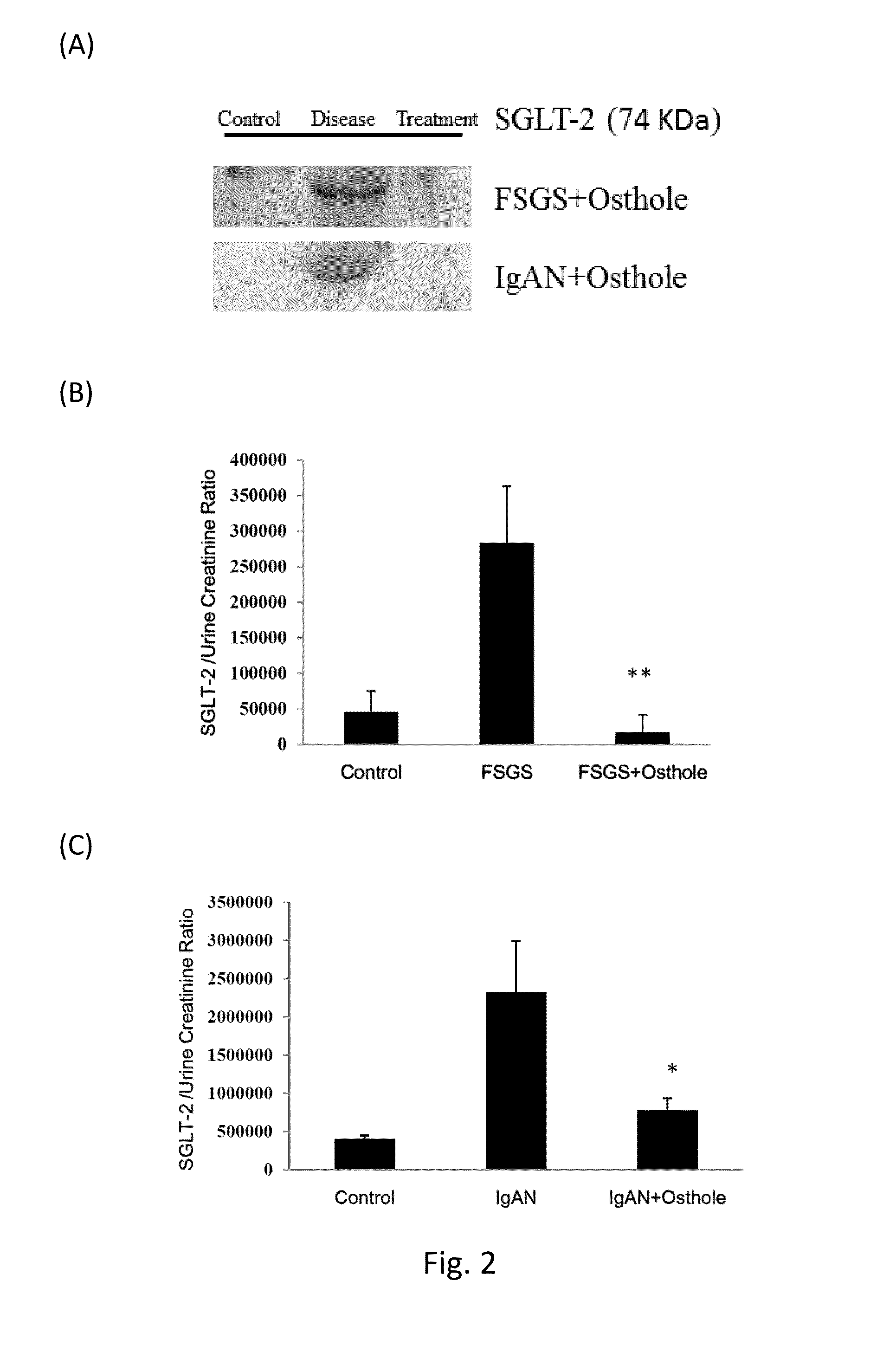
SGLT2 as biomarker for diagnosing chronic kidney disease and monitoring the disease status after treatment
The present invention relates to a method for diagnosis of a chronic kidney disease in a subject by detecting the expression level of a sodium-glucose linked transporter 2 (SGLT2) protein in urine samples. A method for monitoring progression of a chronic kidney disease in a patient is also provided.
Owner:NAT DEFENSE MEDICAL CENT
Inhibitors of sodium glucose co-transporter 2 and methods of their use
ActiveCN101343296BInhibitory activityOrganic active ingredientsSugar derivativesDiabetes mellitusDisease
Owner:LEXICON PHARM INC
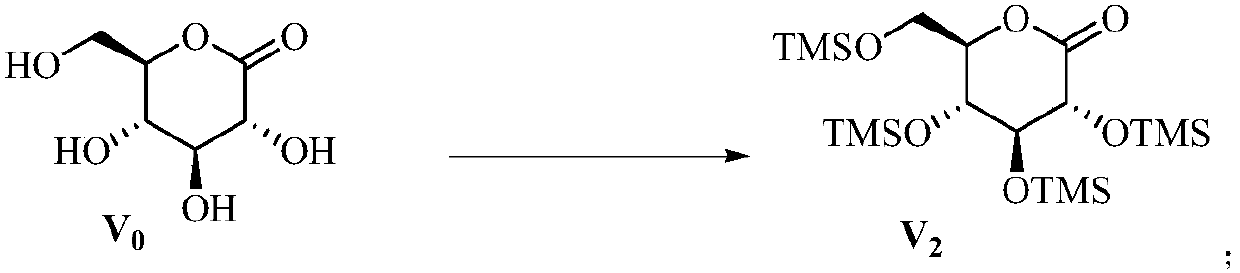
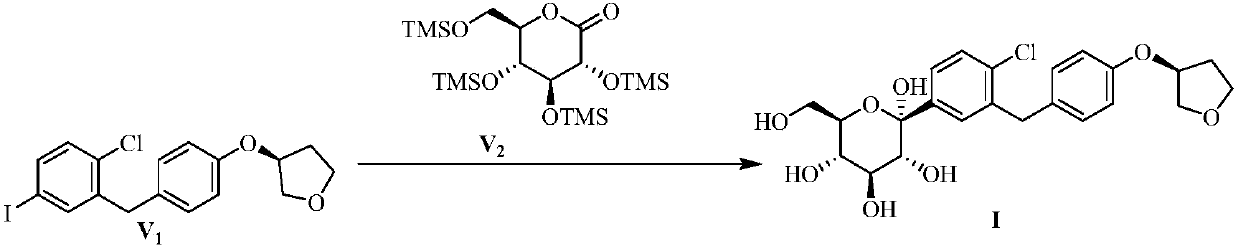
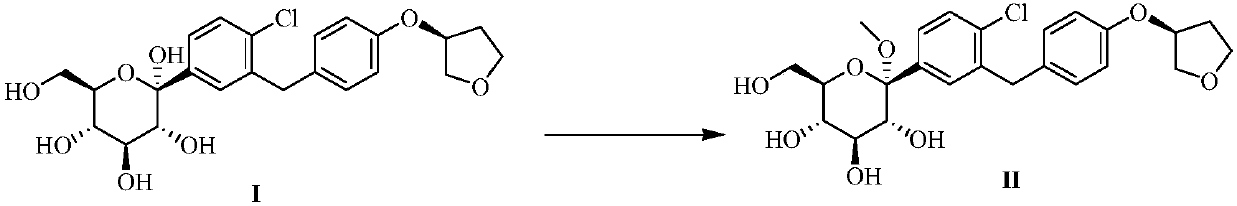
Preparation method of sodium-glucose cotransporter 2 inhibitor
ActiveCN107686496ASaccharide with carbocyclic radicalsSugar derivativesSodium-Glucose Cotransporter 2 InhibitorPalladium
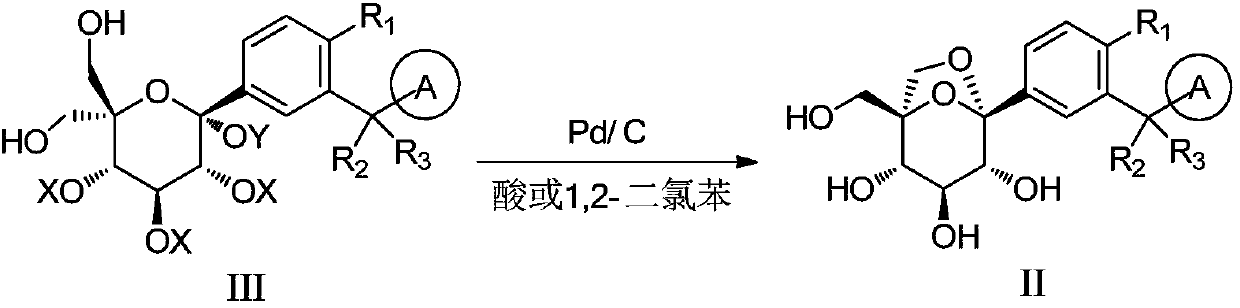
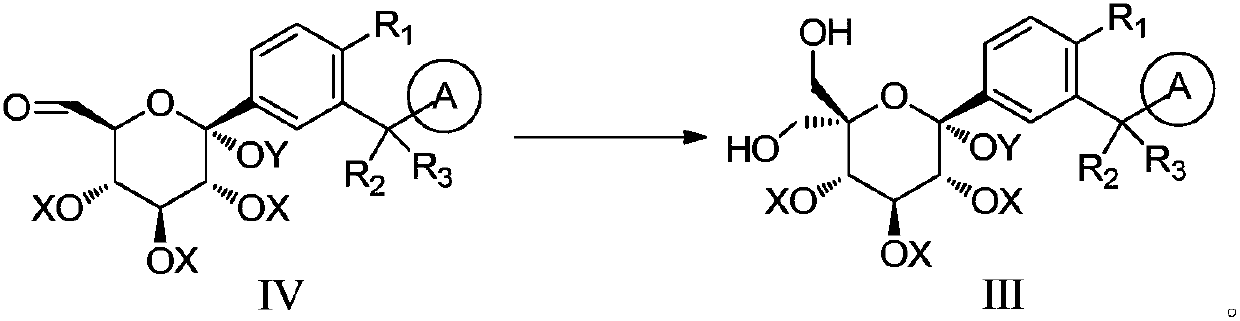
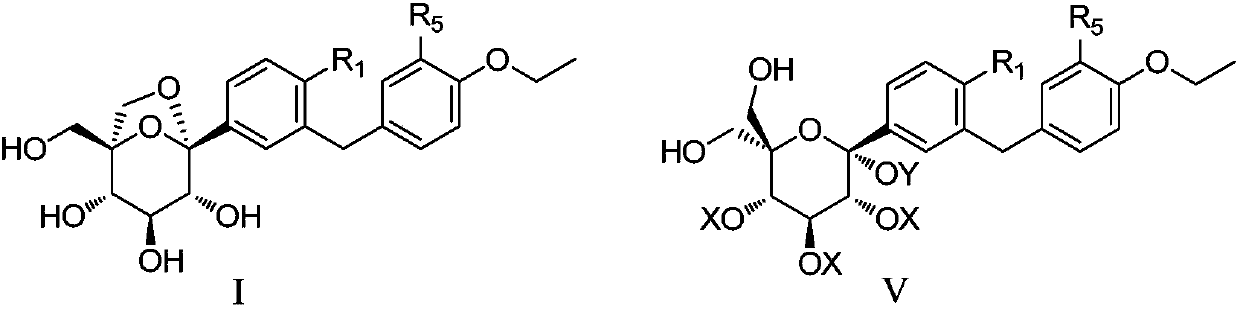
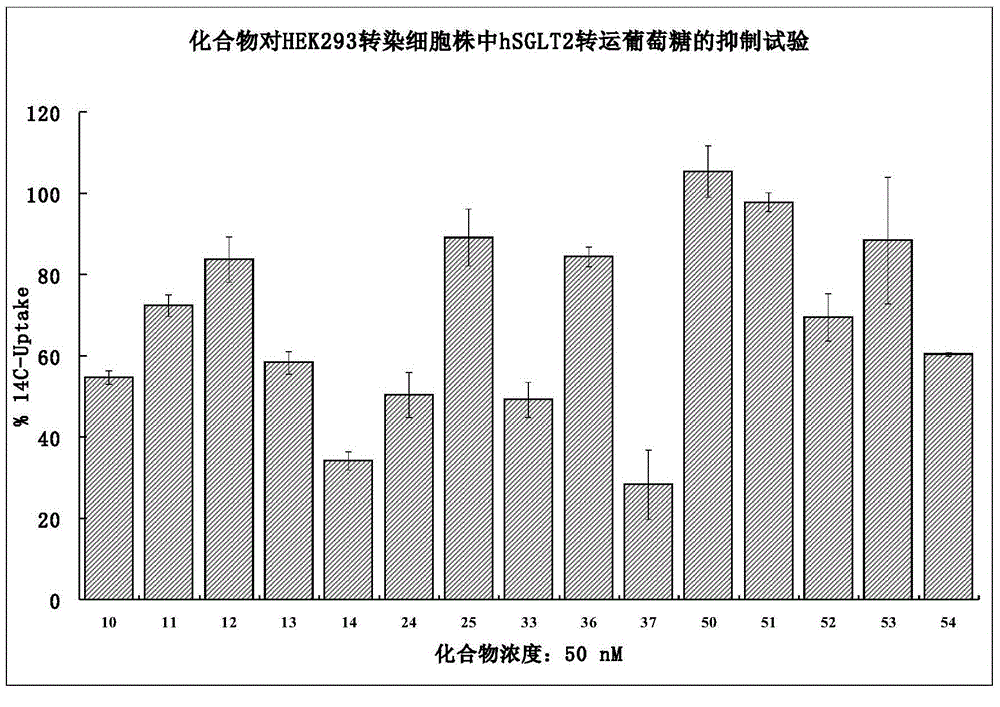
The invention discloses a preparation method of a sodium-glucose cotransporter 2 inhibitor. The preparation method specifically relates to a reaction step for converting a compound of a formula III into a compound of a formula II by a 'one-pot method' in a palladium carbon / acid or 1,2-dichlorobenzene catalytic system, wherein the definition of each substituent in the formula II and the formula IIIare the same as that in the description. The method has simple operation and low cost, and is suitable for large-scale production.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Novel SGLT2 inhibitor compounds and pharmaceutical composition thereof
InactiveCN104672227AGood physical and chemical propertiesLow toxicityOrganic active ingredientsSenses disorderAcute hyperglycaemiaDiabetic retinopathy
The invention discloses novel SGLT2 inhibitor compounds shown in a formula (I), pharmaceutically acceptable salts, easily hydrolyzed pre-drug esters or isomers and a pharmaceutical composition which contains the compounds as an inhibitor of a sodium-glucose co-transporter (SGLT). The pharmaceutical composition is used for treating or delaying related diseases such as diabetes, diabetic retinopathy, diabetic neuropathy, diabetic nephropathy, insulin resistance, hyperglycemia, hyperinsulinemia, rise of fatty acid or glycerinum, hyperlipemia, obesity, hypertriglyceridemia, X syndrome, diabetic complications, atherosclerosis, hypertension and the like. The formula is shown in the description.
Owner:JIANGSU ATOM BIOSCI & PHARMA CO LTD
Agent for treating retinopathy
ActiveUS20190240243A1Easy to eliminateImprove securitySenses disorderMetabolism disorderSGLT2 InhibitorGlycemic
It is to provide an agent for treating and / or ameliorating retinopathy caused by glucose. It is an agent for treating retinopathy caused by glucose comprising sodium / glucose cotransporter2 inhibitor (SGLT2 inhibitor) as an active ingredient, and is used at a normal dosage amount, or at a lower dosage whereby no lowering in blood sugar is observed.
Owner:CARNA HEALTH SUPPORT LLC
Anti-system ASC amino acid transporter 2 (ASCT2) antibody
An object of the present invention is to provide a monoclonal antibody which is useful for treating or diagnosing a disease relating to system ASC amino acid transporter 2 (hereinafter, referred to as “ASCT2”) or a method using the antibody. The present invention provides a monoclonal antibody which specifically recognizes a native three-dimensional structure of an extracellular region of ASCT2 and binds to the extracellular region, or an antibody fragment thereof; a hybridoma which produces the antibody; a DNA which encodes the antibody; a vector which contains the DNA; a transformant obtainable by introducing the vector; a process for producing an antibody or an antibody fragment thereof using the hybridoma or the transformant; and a therapeutic agent using the antibody or the antibody fragment thereof, and a diagnostic agent using the antibody or the antibody fragment thereof.
Owner:KYOWA HAKKO KIRIN CO LTD
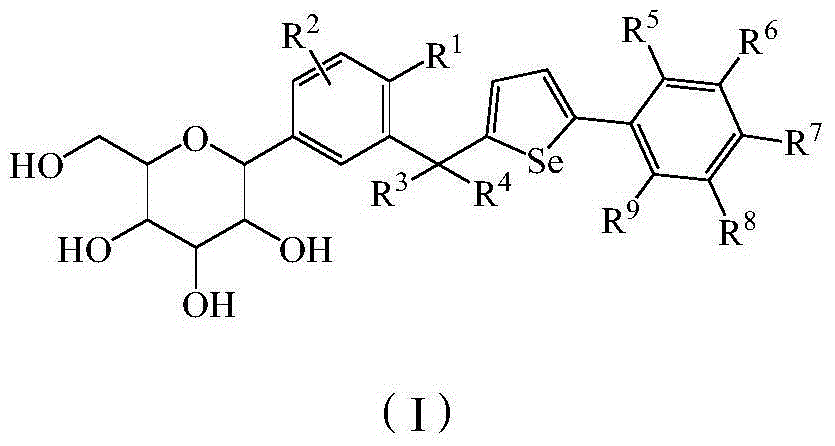
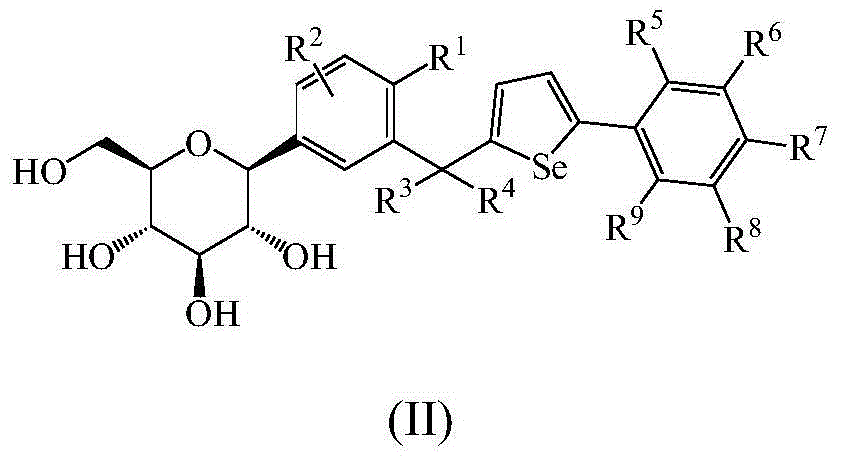
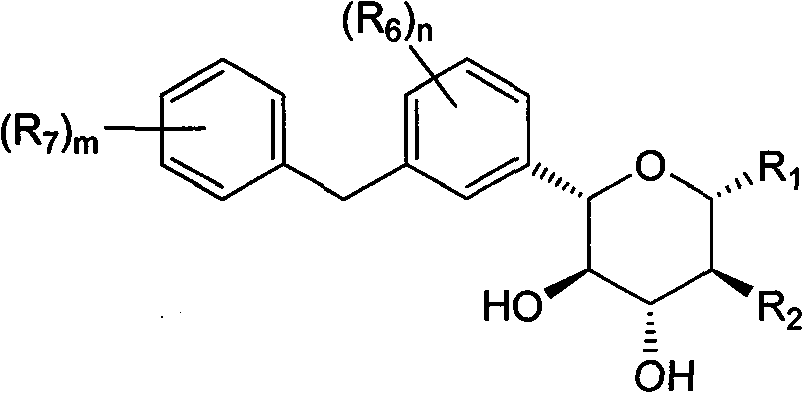
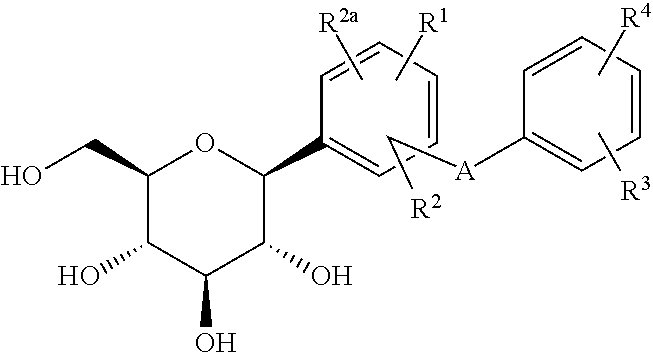
Phenyl C-glucoside derivatives, preparation methods and uses thereof
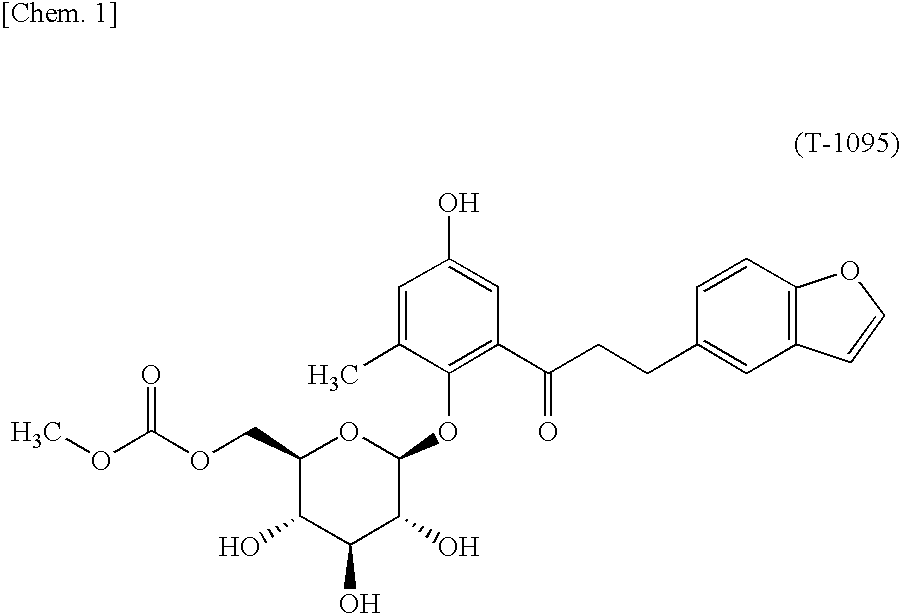
InactiveUS9062087B2High activityBiocideSaccharide with heterocyclic radicalsGlucosideTranslocator protein 2
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
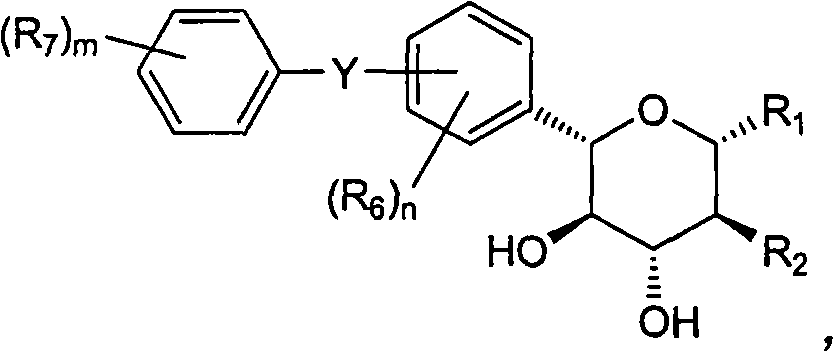
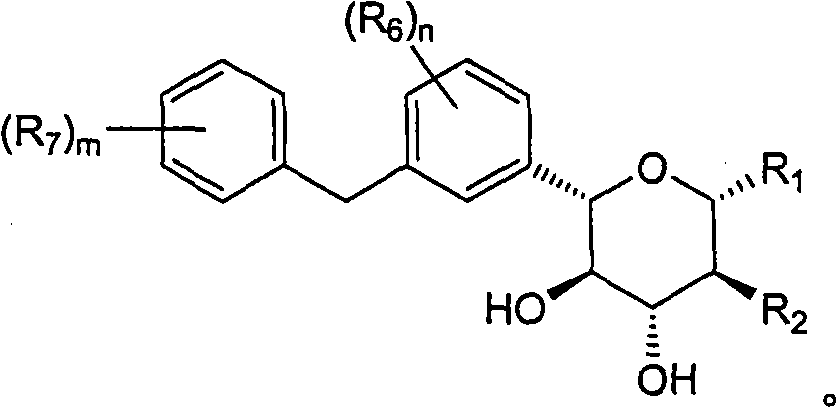
Novel c-aryl glucoside sglt2 inhibitors and pharmaceutical compositions comprising same
A novel C-aryl glucoside compound, or a pharmaceutically acceptable salt or a prodrug thereof having an inhibitory activity against sodium-dependent glucose cotransporter 2 (SGLT2) being present in the intestine and kidney; and a pharmaceutical composition comprising the same as an active ingredient, which is useful for preventing or treating metabolic disorders, particularly, diabetes, are provided.
Owner:THE GREEN CROSS CORP
Progression inhibitor for disease attributed to abnormal accumulation of liver fat
InactiveUS20090286751A1Hindering progressSimple compositionBiocideMetabolism disorderDepressantBULK ACTIVE INGREDIENT
The present invention provides pharmaceutical compositions useful as agents for the inhibition of progression of diseases associated with abnormal accumulation of liver lipids. In particular, the pharmaceutical compositions of the present invention which comprise as an active ingredient a sodium / glucose co-transporter 2 inhibitor are highly suitable as an agent for the inhibition of progression of not only common fatty liver but also non-alcoholic fatty liver disease (NAFL), non-alcoholic steatohepatitis (NASH), hypernutritive fatty liver, diabetic fatty liver, alcoholic fatty liver disease toxic fatty liver or the like.
Owner:KISSEI PHARMA
Method for suppressing glucagon secretion of SGLT2 inhibitor
Methods are provided for avoiding an increase in glucagon secretion associated with the administration of a sodium glucose co-transporter 2 (SGLT2) inhibitor via the co-administration of a dipeptidyl peptidase IV (DPP IV) inhibitor. Additionally, methods are provided for normalizing the glucagon secretion associated with the administration of a sodium glucose co-transporter 2 (SGLT2) inhibitor via the co-administration of a dipeptidyl peptidase IV (DPP IV) inhibitor. The present invention also relates to methods for treating diabetes, especially type 2 diabetes, as well as hyperglycemia, hyperinsulinemia, obesity, hypertriglyceridemia, syndrome X, diabetic complications, atherosclerosis and related diseases, with the method comprising administering the SGLT2 inhibitor and the dipeptidyl peptidase IV (DPP IV) inhibitor.
Owner:ASTRAZENECA AB
Drug compound containing sodium-glucose synergic transport protein 2 inhibitor
ActiveCN106955273AEnhanced inhibitory effectSimple preparation processOrganic active ingredientsMetabolism disorderDissolutionMethyl group
The invention discloses a drug compound containing a sodium-glucose synergic transport protein 2 inhibitor. Specifically, the drug compound contains 1,6-dehydrated-1-C-{4-chlorine-3-[(3-fluorine-4-phenetyl) methyl] phenyl}-5-C-(hydroxymethyl)-beta-L-idose or a compound formed by the same with amino acid, pre-gelled starch and at least one pharmaceutically acceptable excipient. The drug compound disclosed by the invention has the characteristics of strong technical operability and quick and complete dissolution.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Novel c-aryl ansa sglt2 inhibitors
ActiveUS20140213642A1Prevention and/or treatment of metabolic disordersBiocideOrganic active ingredientsIntestinal structureDiabetes mellitus
Disclosed is a novel C-aryl ansa compound having an inhibitory activity against sodium-dependent glucose cotransporter 2 (SGLT2) being present in the intestine and kidney, and a pharmaceutical composition comprising the same as an active ingredient, which is useful for preventing or treating metabolic disorders, particularly diabetes. Also provided are a method for preparing the compound, and a method for preventing or treating metabolic disorders, particularly diabetes, by using the compound.
Owner:THE GREEN CROSS CORP
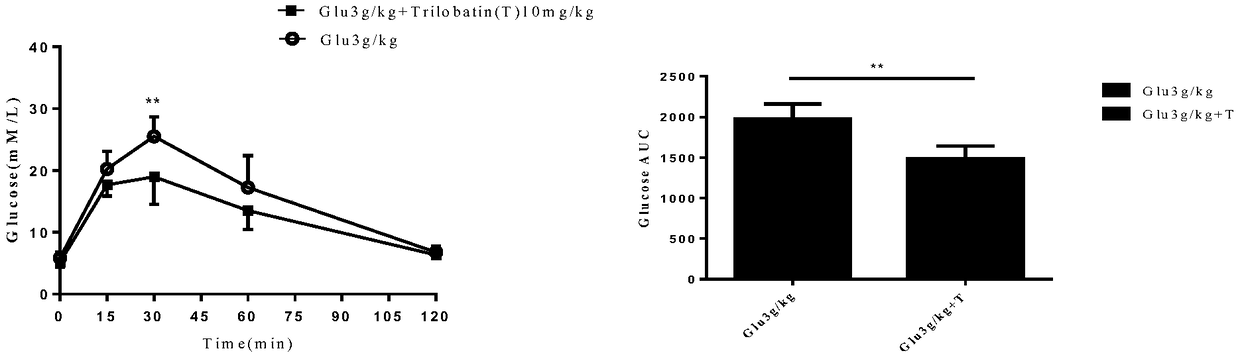
Sodium-glucose cotransporter 2 (SGLT2) inhibitor and application thereof
InactiveCN109456370AInhibition of reabsorptionLower blood sugar levelsSugar derivativesMetabolism disorderSGLT2 InhibitorGlycemic
The invention provides a sodium-glucose cotransporter 2 (SGLT2) inhibitor. The SGLT2 inhibitor is trilobatin, and the specific structure of the trilobatin is shown as the following formula (please seethe specifications for the formula); and the trilobatin and the SGLT2 interact with each other, the trilobatin is in hydrogen bond connection with ASn75 and Tyr290 sites of the SGLT2, and thus the trilobatin can significantly inhibit glucose reabsorption, lowers the overall blood glucose level of an experimental subject, and meanwhile, can further improve insulin resistance of the experimental subject effectively.
Owner:CHONGQING UNIV OF TECH
L-proline complex of sodium-glucose cotransporter 2 inhibitor, monohydrate and crystal form thereof
InactiveUS10301344B2High melting pointImprove stabilityOrganic active ingredientsSugar derivativesSolventChemical stability
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Thiophene derivative as SGLT2 inhibitor and pharmaceutical composition comprising same
ActiveUS9006187B2Preventing and treating diabetesEffective inhibitorBiocideSaccharide with heterocyclic radicalsIntestinal structureThiophene derivatives
The present invention relates to a novel compound with thiophene ring having an inhibitory activity against sodium-dependent glucose cotransporter 2 (SGLT2) being present in the intestine and kidney, and a pharmaceutical composition comprising the same as an active ingredient, which is useful for preventing or treating metabolic disorders, particularly diabetes. The prevention also provides a method for preparing same, a pharmaceutical composition containing same, and a method for preventing or treating metabolic disorders, particularly diabetes.
Owner:THE GREEN CROSS CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Substituted 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-ol compounds and methods relating thereto Substituted 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-ol compounds and methods relating thereto](https://images-eureka.patsnap.com/patent_img/a0bd7133-284f-4ef7-95fe-2ba89ead4c06/US20080167337A1-20080710-D00001.png)
![Substituted 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-ol compounds and methods relating thereto Substituted 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-ol compounds and methods relating thereto](https://images-eureka.patsnap.com/patent_img/a0bd7133-284f-4ef7-95fe-2ba89ead4c06/US20080167337A1-20080710-D00002.png)
![Substituted 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-ol compounds and methods relating thereto Substituted 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-ol compounds and methods relating thereto](https://images-eureka.patsnap.com/patent_img/a0bd7133-284f-4ef7-95fe-2ba89ead4c06/US20080167337A1-20080710-D00003.png)



![1-(beta-d-pyranoglucosyl)-4-methyl-3-[5-(4-fluorophenyl)-2-thienylmethyl]benzene and l-phenylalanine eutectic compound and preparation method thereof 1-(beta-d-pyranoglucosyl)-4-methyl-3-[5-(4-fluorophenyl)-2-thienylmethyl]benzene and l-phenylalanine eutectic compound and preparation method thereof](https://images-eureka.patsnap.com/patent_img/6e791490-d6a2-4dbd-9497-624e0cd288bb/HDA00002773789200011.png)
![1-(beta-d-pyranoglucosyl)-4-methyl-3-[5-(4-fluorophenyl)-2-thienylmethyl]benzene and l-phenylalanine eutectic compound and preparation method thereof 1-(beta-d-pyranoglucosyl)-4-methyl-3-[5-(4-fluorophenyl)-2-thienylmethyl]benzene and l-phenylalanine eutectic compound and preparation method thereof](https://images-eureka.patsnap.com/patent_img/6e791490-d6a2-4dbd-9497-624e0cd288bb/FDA00002773789000011.png)
![1-(beta-d-pyranoglucosyl)-4-methyl-3-[5-(4-fluorophenyl)-2-thienylmethyl]benzene and l-phenylalanine eutectic compound and preparation method thereof 1-(beta-d-pyranoglucosyl)-4-methyl-3-[5-(4-fluorophenyl)-2-thienylmethyl]benzene and l-phenylalanine eutectic compound and preparation method thereof](https://images-eureka.patsnap.com/patent_img/6e791490-d6a2-4dbd-9497-624e0cd288bb/FDA00002773789000012.png)