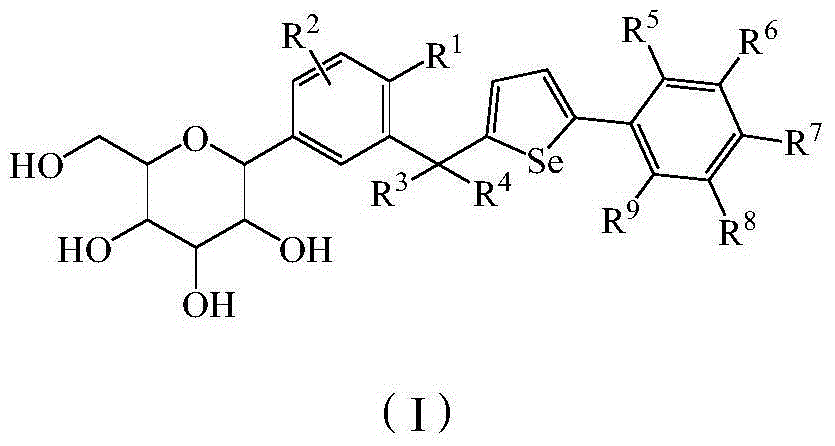
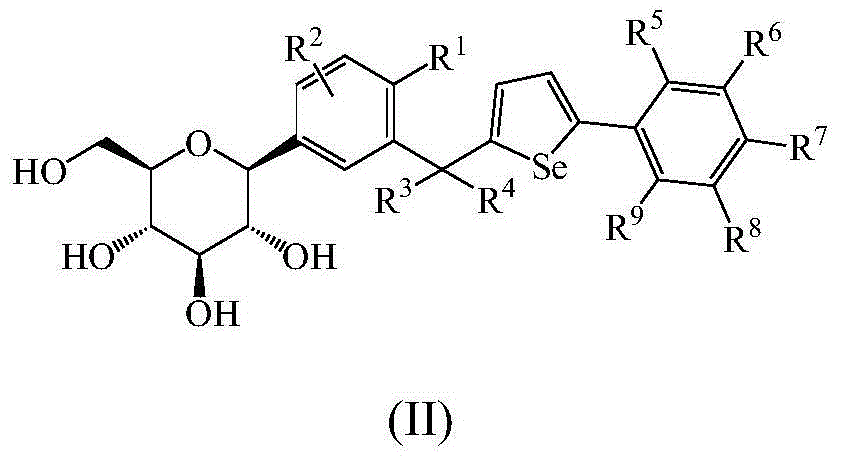
Glucoside derivatives and pharmaceutical compositions thereof
A kind of drug and compound technology, applied in the field of a class of glucoside derivatives and pharmaceutical compositions thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
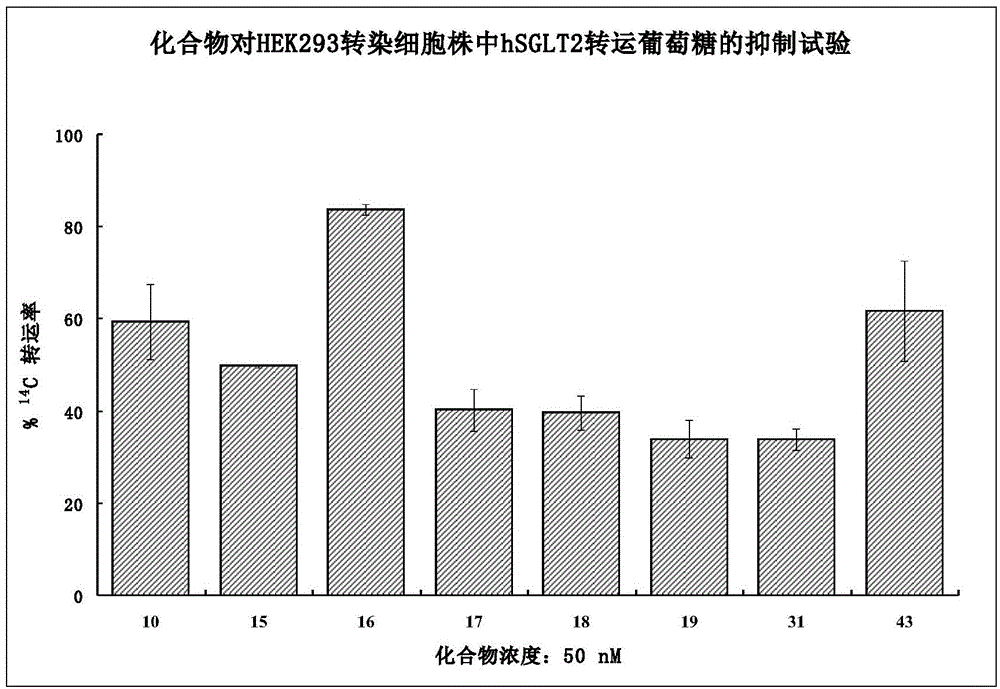
[0069] (2S,3R,4R,5S,6R)-2-{3-{Deutero[5-(4-fluorophenyl)selenophen-2-yl]methyl}-4-methylphenyl}-6 Synthesis of -(Hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol (10)
[0070]
[0071] Step A: D-gluconolactone (5.0g, 28.1mmol) and N-methylmorpholine (23.3g, 222mmol) were dissolved in THF (50mL), and trimethylchlorosilane (18.3 g, 168 mmol). Stirring was continued at this temperature for 1 hour after the addition was complete, and then the mixture was naturally warmed to room temperature and stirred overnight. Toluene (200 mL) was added, and ice water (200 mL) was added dropwise under an ice-water bath to control the inner temperature not to exceed 10°C. Separate the layers, collect the organic layer, and extract the aqueous layer with toluene (50 mL). The combined organic layers were washed successively with saturated aqueous sodium dihydrogen phosphate (70 mL×2), water (50 mL), saturated brine (50 mL), and dried over anhydrous sodium sulfate. The solvent was distilled off...
Embodiment 2
[0082] (2S,3R,4R,5S,6R)-2-{3-{Dideutero[5-(4-fluorophenyl)selenophen-2-yl]methyl}-4-methylphenyl}- Synthesis of 6-(Hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol (15)
[0083]
[0084]Step A: Dissolve compound 4 (300mg, 0.639mmol) in anhydrous THF (10mL), add sodium borodeuteride (80mg, 1.91mmol), then add boron trifluoride diethyl ether (364mg, 2.56mmol) dropwise in an ice-water bath ). Stirring was continued at this temperature for 20 minutes after the addition was complete, then the temperature was raised to room temperature and stirred for 1 hour, and then the temperature was raised to reflux and stirred overnight. Saturated brine (20 mL) was added, extracted with dichloromethane (15 mL×3), the combined organic layers were washed successively with saturated aqueous sodium bicarbonate (15 mL) and saturated brine (10 mL), and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure, and the product was purified by column chromatography (200-...
Embodiment 3
[0087] (2S,3R,4R,5S,6R)-2-{3-{Deuterated[5-(4-fluorophenyl)selenophen-2-yl]methyl}-4-chlorophenyl}-6- Synthesis of (Hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol (16)
[0088]
[0089] For the preparation method of compound 16, refer to Example 1, wherein 2-methyl-5-iodobenzoic acid in step D of Example 1 is replaced by 2-chloro-5-iodobenzoic acid. 1 H NMR (CD 3 OD, 300MHz) δ7.51-7.46(m, 3H), 7.39-7.36(m, 1H), 7.33-7.30(m, 1H), 7.24-7.23(m, 1H), 7.07-7.02(m, 2H) , 6.98-6.97(m, 1H), 4.31-4.27(m, 1H), 4.14-4.11(m, 1H), 3.89-3.85(m, 1H), 3.72-3.67(m, 1H), 3.43-3.39( m, 4H). MS (EI, m / z): 536.1 [M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com