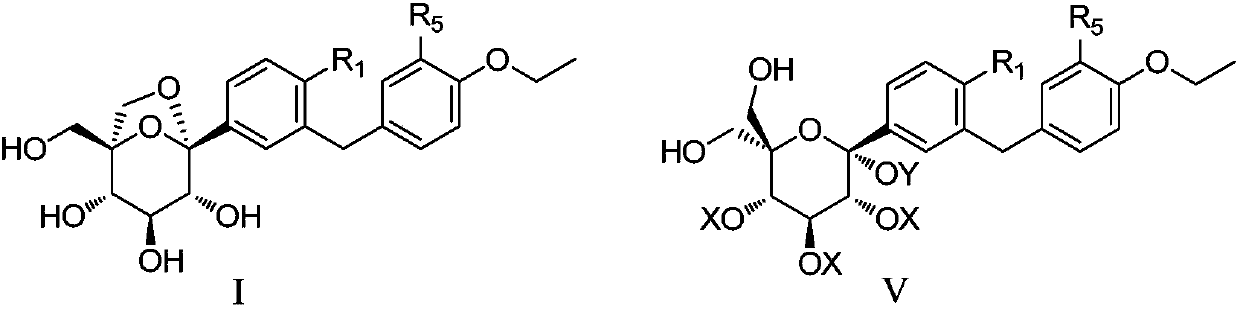
Preparation method of sodium-glucose cotransporter 2 inhibitor
A compound and halogen technology, applied in the field of preparation of sodium-glucose cotransporter 2 inhibitors and their derivatives, can solve the problems of complex compound structure, long synthetic route, and great influence on process yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Compound III-1 was obtained by reaction under paraformaldehyde / potassium hydroxide conditions
[0052]
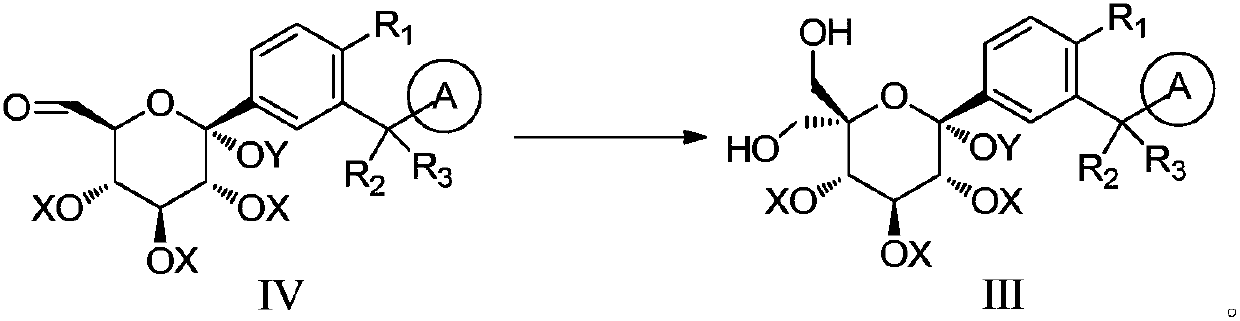
[0053] 10 g of compound IV-1, 56.5 g of 1, 4-dioxane was put into the reaction bottle, stirred and dissolved. Add 2.6g of paraformaldehyde and 3.9g of potassium hydroxide in turn under the protection of argon. After the addition is complete, raise the temperature to 20-30°C and keep it for 30 minutes, then raise the temperature to 50-55°C and keep it for 2 hours. Cool down to room temperature, filter, and concentrate the filtrate to dryness under reduced pressure, add 30g of purified water and 40g of dichloromethane, extract and separate the organic phase, wash with saturated brine (24g×2), separate layers, concentrate the organic layer under reduced pressure, and use Separation and purification on a silica gel column (eluent: petroleum ether / dichloromethane) gave compound III-1 as an oil, which was slurried by adding isopropyl ether to get 5.8 g of com...
Embodiment 2
[0054] Embodiment 2: "one pot method" synthetic compound I-1
[0055]
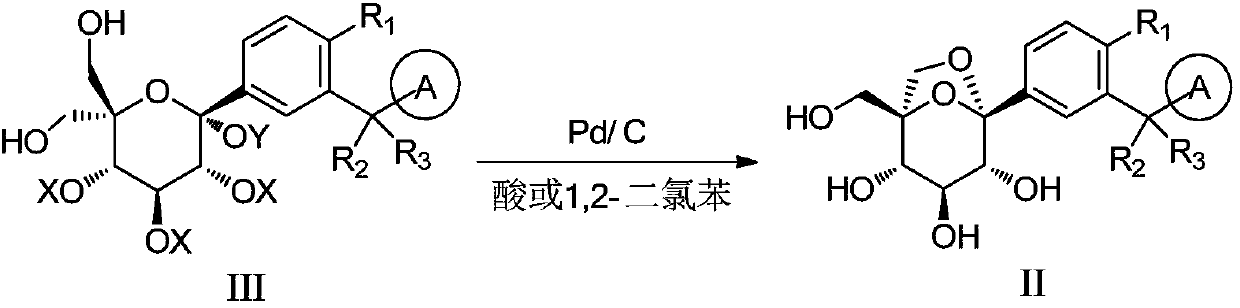
[0056] In the reaction bottle, put 50g of compound III-1, 300mL of tetrahydrofuran and 250mL of methanol, stir to dissolve, control the temperature below 30°C, add 8.8mL of hydrochloric acid and 2.5g of 10% palladium carbon, and then add 50mL of methanol to rinse the palladium carbon. After the addition is completed, the hydrogen gas is replaced three times, and the hydrogen gas is passed under normal pressure, and the temperature is controlled at 30±5°C for 8 hours, and the sampling is started and controlled until the reaction ends in accordance with the regulations. Filter the reaction solution through diatomaceous earth, wash the filter cake with 150mL of tetrahydrofuran until the washing solution meets the requirements, adjust the pH of the filtrate to neutral with saturated sodium bicarbonate solution, concentrate under reduced pressure, add 320mL of ethyl acetate to dissolve, add Stir and extract ...
Embodiment 3
[0057] Embodiment 3: "one pot method" synthetic compound I-1
[0058] 50 g of compound III-1, 237.5 g of methanol, and 267.5 g of tetrahydrofuran were put into the reaction flask, stirred and dissolved. Then add 50 g of 1,2-dichlorobenzene, and stir evenly. Add 10% palladium on carbon (25g, W:W=50%), after the addition is complete, replace with hydrogen three times, and react with hydrogen at normal pressure and temperature for 8h, monitor by TLC until the raw material point disappears, and terminate the reaction. Filter, wash with tetrahydrofuran (100g×3), concentrate the filtrate to dryness, dissolve the residue with 500g ethyl acetate, wash with water (400g×3), separate layers, concentrate the organic layer to dryness, and separate and purify on a silica gel column (eluent: dichloromethane / methanol) to obtain 26 g of compound I-1 with a yield of 86.6% and a purity of 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com