Drug compound containing sodium-glucose synergic transport protein 2 inhibitor
A composition and drug technology, applied in the field of pharmaceutical preparations, to achieve the effect of excellent protein inhibition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
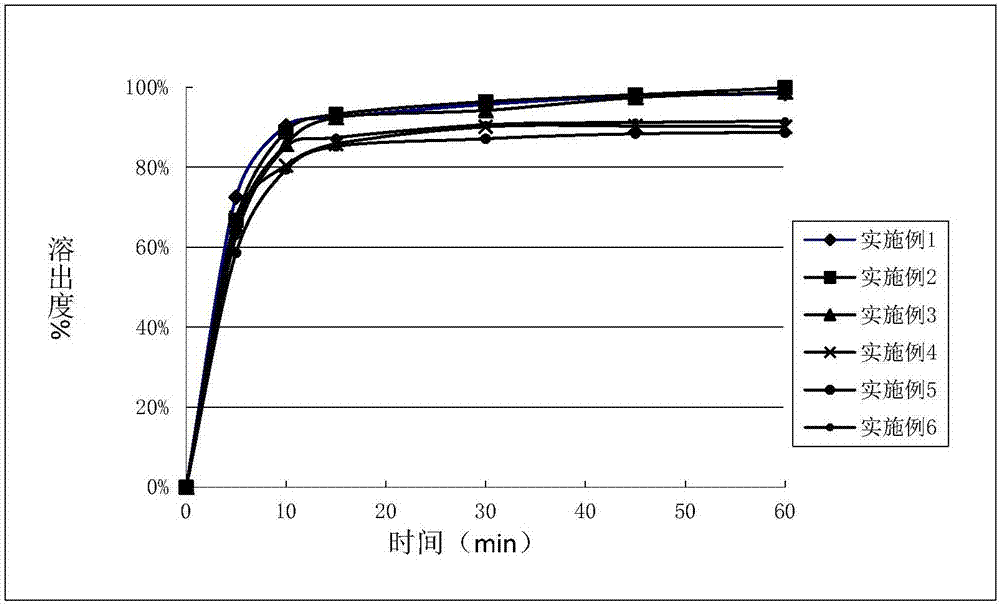
Embodiment 1 to 6
[0040] Mix compound A, mannitol, lactose, pregelatinized starch, microcrystalline cellulose, and croscarmellose sodium according to the ratio in Table 1, and moisten with 10% cross-linked polyvinylpyrrolidone aqueous solution The aerosol is subjected to wet granulation and tablet compression to prepare tablets.
[0041] Table 1
[0042] Element Example 1 Example 2 Example 3 Example 4 Example 5 Example 6 Complex A 1.25 15 60 18 50 50 Mannitol — — — 95.52 72.64 — lactose — — — — — 77.55 pregelatinized starch 53.39 97.48 65.6 — — — microcrystalline cellulose 26 48.8 32.0 47.76 35.0 30.0 CCNa 3.6 7.2 10.8 7.2 10.8 10.8 PVP K30 4.86 9.72 9.8 9.72 9.76 9.85 purified water 43.74 87.48 88.2 87.48 87.84 88.65 Magnesium stearate 0.9 1.8 1.8 1.8 1.8 1.8 total 90 180 180 180 180 180
[0043] Unit: mg
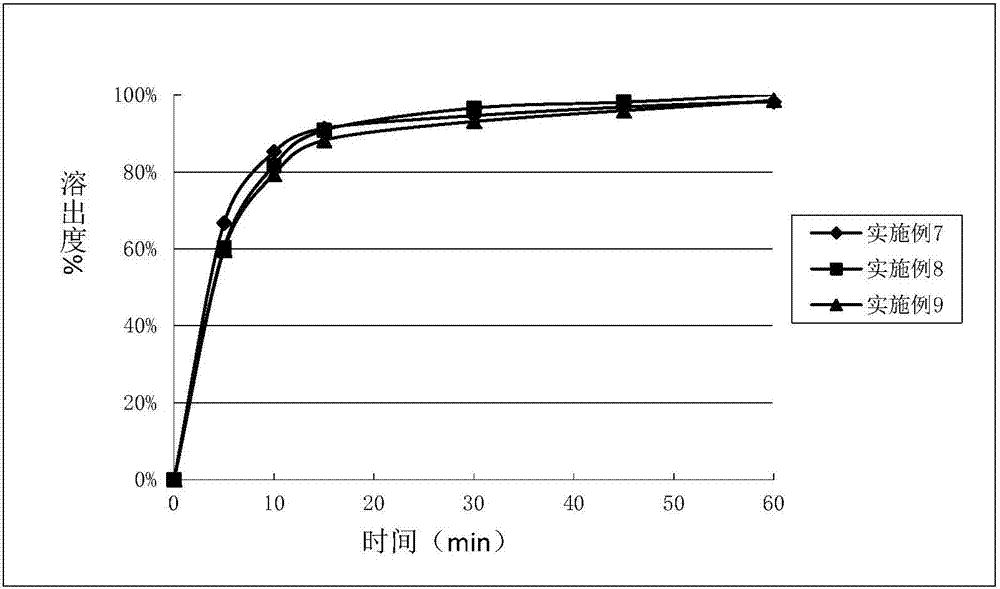
Embodiment 7~9
[0045] With the prescription ratio of Table 2, compound A, pregelatinized starch, microcrystalline cellulose, sodium starch glycolate are mixed evenly, respectively with 10% polyvinylpyrrolidone, 10% hydroxypropyl cellulose and 4% The aqueous solution of hydroxypropyl methylcellulose was used as a wetting agent, and wet granulation was carried out, and tableted to prepare the tablets of Examples 7-9.
[0046] Table 2
[0047] Element Example 7 Example 8 Example 9 Complex A 20 20 20 pregelatinized starch 91.86 92.19 95.9 microcrystalline cellulose 46.0 46.0 48.0 Sodium carboxymethyl starch 10.8 10.8 10.8 PVP K30 9.54 — — HPC — 9.21 — HPMC E5 — — 3.5 purified water 85.86 82.89 84.0 Magnesium stearate 1.8 1.8 1.8 total 180 180 180
[0048] Unit: mg
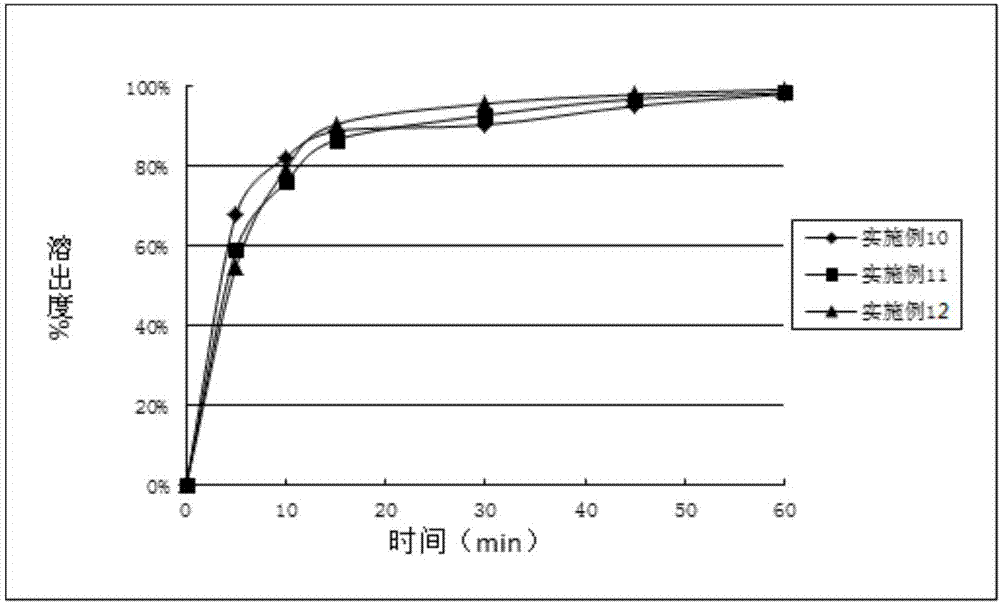
Embodiment 10~12
[0050] Mix compound A, pregelatinized starch, microcrystalline cellulose, and crospovidone evenly with the prescription ratio in Table 3, and use 10% crospovidone and 10% hydroxypropyl cellulose as moisteners respectively. Wet agent, carry out wet granulation, tabletting, prepare the tablet of embodiment 10~12.
[0051] table 3
[0052] Element Example 10 Example 11 Example 12 Complex A 2.5 20 70 pregelatinized starch 51.5 91.96 63.08 microcrystalline cellulose 25.7 45.9 25 Crospovidone 5.4 10.8 10.8 PVP K30 4.86 — — HPC — 9.54 9.32 purified water 43.74 85.86 83.88 talcum powder 0.9 1.8 1.8 total 90 180 180
[0053] Unit: mg
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com