Method for catalytic synthesis of dicoumarin compound through lipases
A technology of dicoumarin and lipase, which is applied in the field of biocatalysis, can solve the problems of difficult catalyst preparation, difficult recycling, high reaction temperature, etc., and achieve the effects of wide application range of substrates, high catalytic activity, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
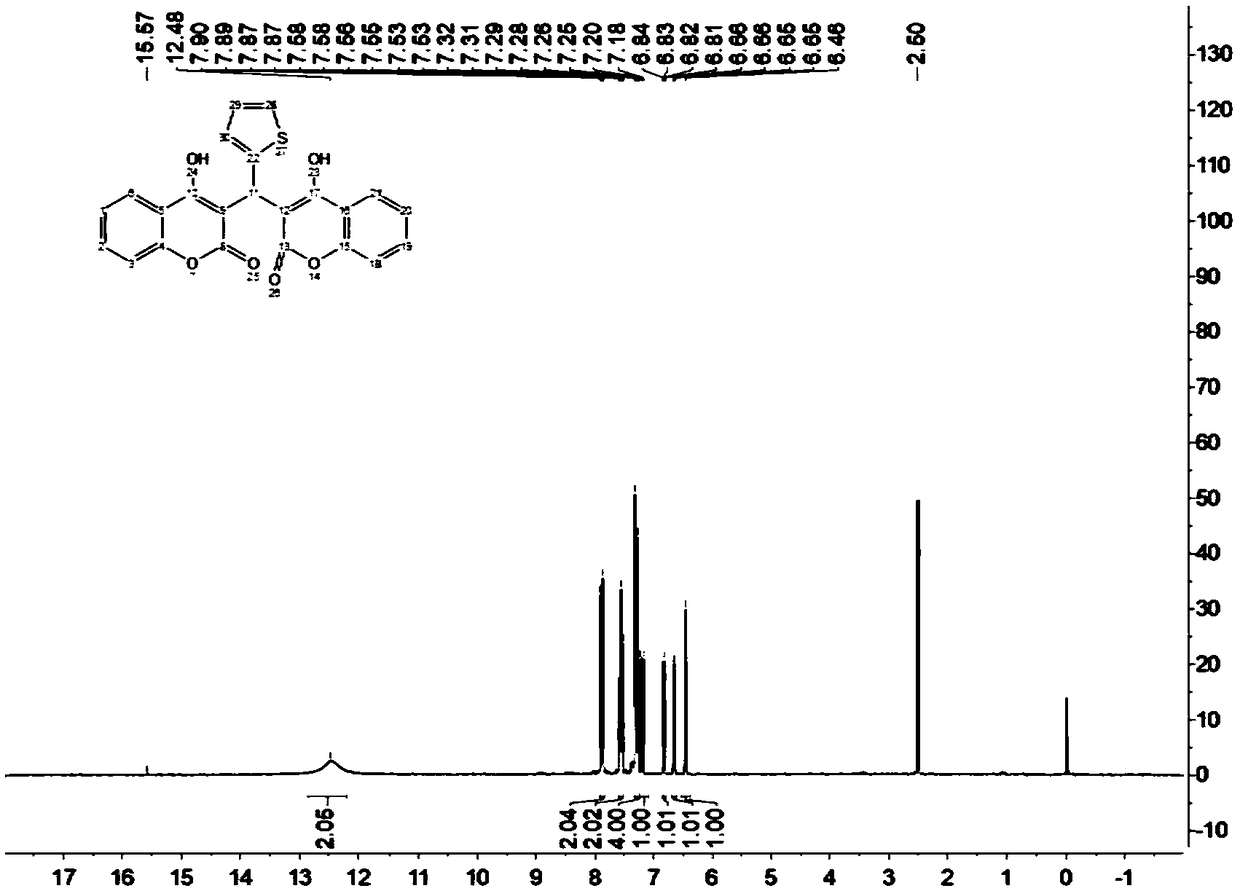
[0037] Add 1mmol of p-chlorobenzaldehyde and 2mmol of 4-hydroxycoumarin into a 10mL reaction flask, then add 50mg of lipase RMIM, 5mL of water, stir the reaction at 45°C, TLC (n-hexane / ethyl acetate, 1 / 2, v / v) Monitoring the progress of the reaction. After 20 hours, filter and recover the water, add 1,4-epoxyhexacycline to the filter residue to dissolve and filter to recover the enzyme, reduce the pressure and rotate the filtrate to recover the 1,4-epoxyhexane ring, wash the filter residue with cold ethanol to obtain the crude product, and use the crude product Hot ethanol recrystallization to obtain the purified target product, the target product is a white solid, the yield is 93%, mp: 257-258 ° C; 1 H NMR (400MHz, DMSO-d 6 )δ11.54(s,1H),11.32(s,1H),8.07(d,J=7.8Hz,1H),7.99(d,J=7.8Hz,1H),7.67–7.60(m,2H), 7.47–7.34 (m, 4H), 7.30 (s, 1H), 7.26 (s, 1H), 7.15 (d, J=7.8Hz, 2H), 6.04 (s, 1H).
Embodiment 2
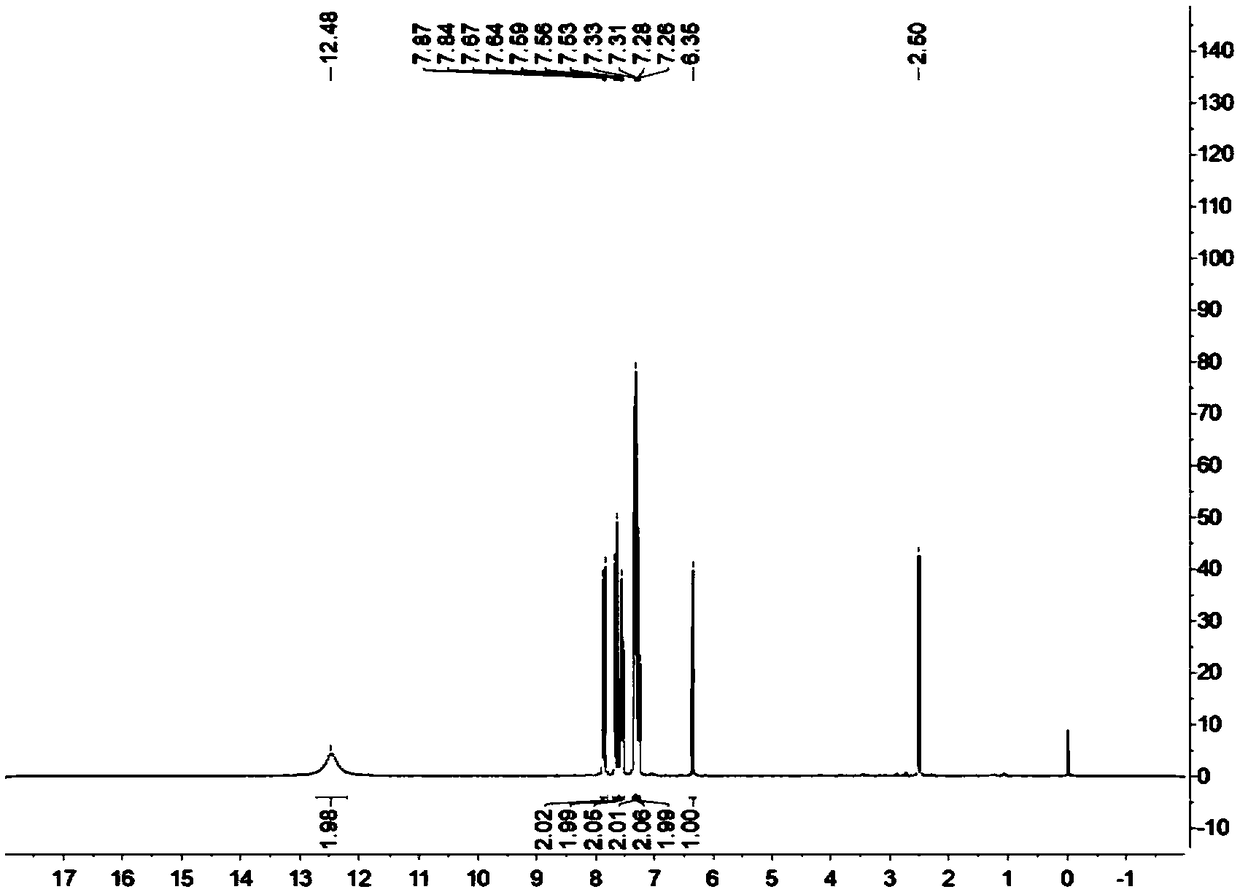
[0039] Add 1mmol benzaldehyde and 2mmol 4-hydroxycoumarin to a 10mL reaction flask, then add 150mg lipase RMIM, 5mL water, stir the reaction at 65°C, TLC (n-hexane / ethyl acetate, 1 / 2, v / v ) to monitor the progress of the reaction. After 2 hours, filter and recover the water, add 1,4-epoxyhexacycline to the filter residue to dissolve and filter to recover the enzyme, reduce the pressure and rotate the filtrate to recover the 1,4-epoxyhexane ring, wash the filter residue with cold ethanol to obtain the crude product, and use Hot ethanol recrystallization to obtain the purified target product, the target product is a white solid, the yield is 89%, m.p.: 231.9-232.3 ° C; 1 H NMR (400MHz, DMSO-d 6 )δ7.91–7.85(m,2H),7.62–7.54(m,2H),7.42–7.26(m,4H),7.26–7.18(m,2H),7.14(d,J=7.4Hz,3H) ,6.35(s,1H).
Embodiment 3
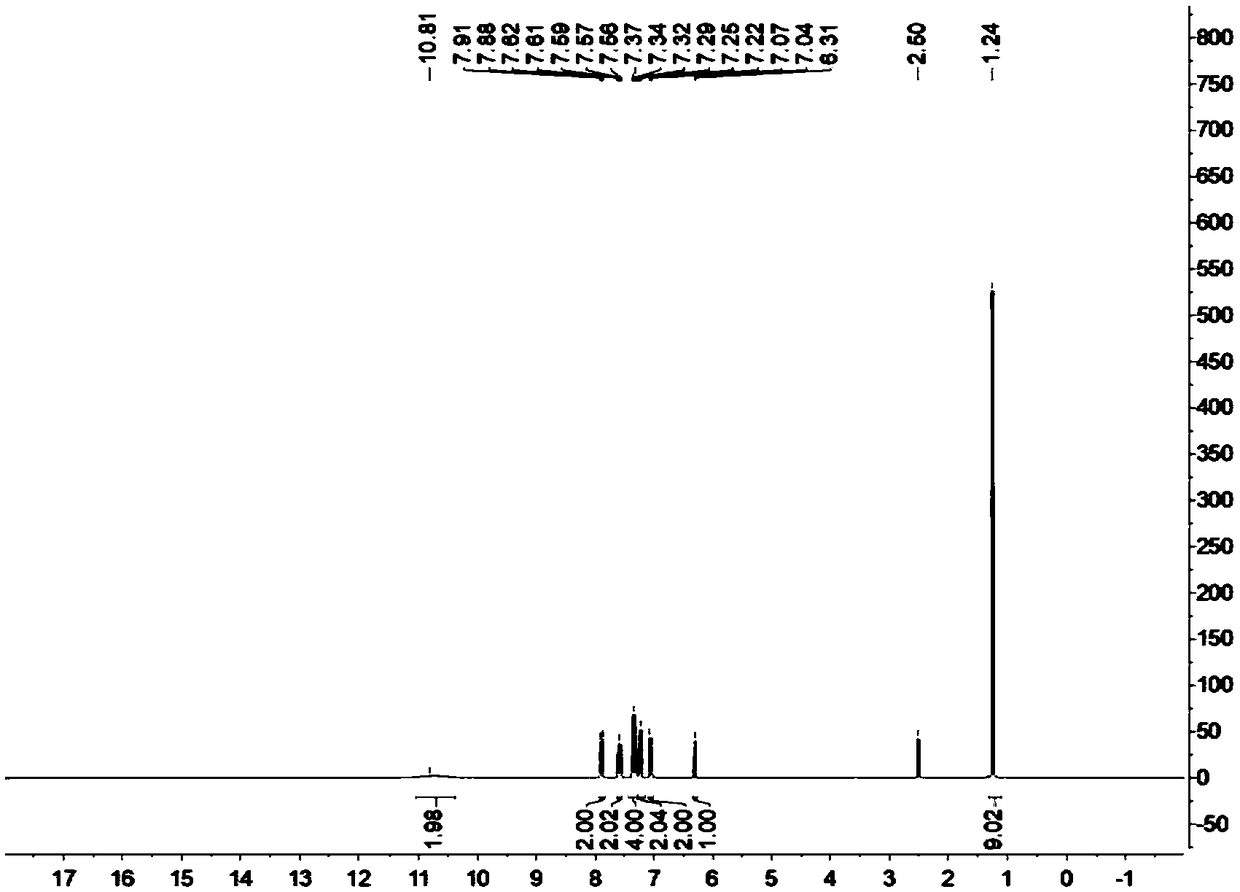
[0041] Add 1mmol p-methoxybenzaldehyde and 2mmol4-hydroxycoumarin to a 10mL reaction flask, then add 5mg lipase RMIM, 5mL water, stir the reaction at 25°C, TLC (n-hexane / ethyl acetate, 1 / 2 , v / v) to monitor the progress of the reaction. After 48 hours, filter and recover water, add 1,4-epoxyhexacycline to the filter residue to dissolve and filter to recover the enzyme, reduce pressure and rotate the filtrate to recover 1,4-epoxyhexane ring, wash the filter residue with cold ethanol to obtain the crude product, and use Hot ethanol recrystallization to obtain the purified target product, the target product is a white solid, the yield is 96%, m.p.: 249-250 ° C; 1 H NMR (400MHz, DMSO-d 6 )δ11.05(s,1H),7.89(dd,J=7.9,1.4Hz,2H),7.64–7.52(m,2H),7.36(d,J=8.0Hz,2H),7.34–7.27(m , 2H), 7.05 (d, J=8.1Hz, 2H), 6.79 (d, J=8.8Hz, 2H), 6.28 (s, 1H), 3.70 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com