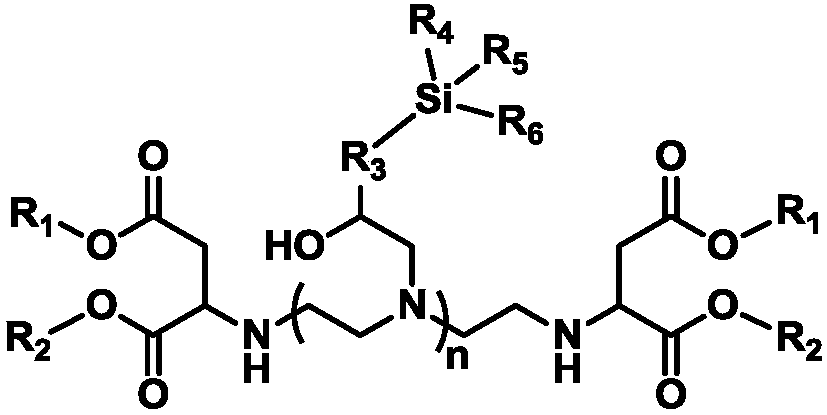
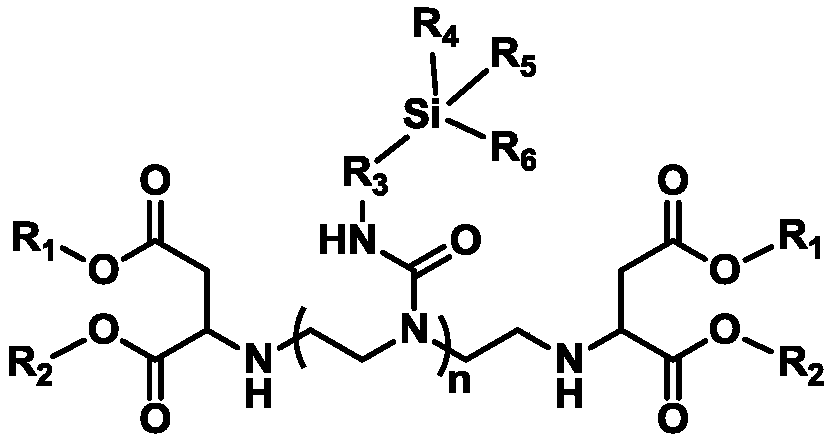
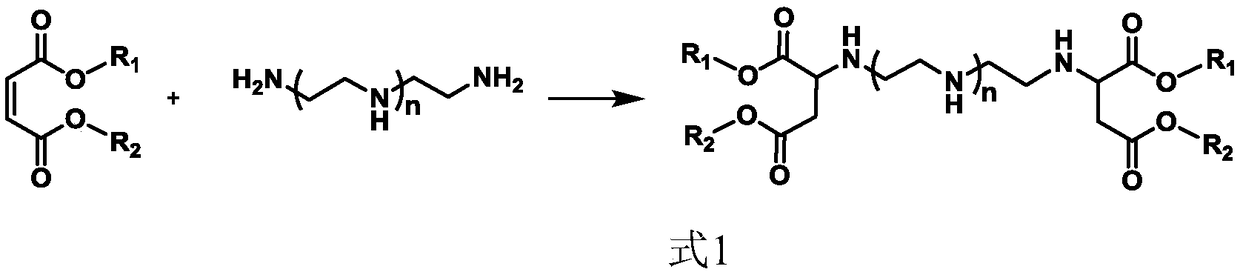
Siloxane-modified aspartic ester as well as preparation method and application thereof
An aspartate and siloxane modification technology, which is applied in chemical instruments and methods, compounds of Group 4/14 elements of the periodic table, organic chemistry, etc., can solve the problems of poor system compatibility and excessive increase in product viscosity. Fast, poor uniformity and other problems, to achieve the effect of high crosslinking density, improved mechanical and physical strength, and excellent substrate adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Add 103.17g (1mol) of diethylenetriamine into a 1L four-neck round bottom flask equipped with a mechanical stirring paddle, a thermometer, a constant pressure dropping funnel, and a N 2 Air line pipe, bubbler, add 344.36g (2mol) diethyl maleate into the constant pressure dropping funnel, pass N into the system 2 10min, replace the air in the system; slowly drop diethyl maleate into the flask under the condition of stirring at 25°C, drop it at a constant speed for 1h, control the reaction temperature at 30°C, then raise the temperature to 60°C for 3h, pass thiol- The unsaturation value in the reaction system measured by iodine titration method was 0.33 mg maleic acid / g resin, indicating that the reaction conversion rate of maleic acid ester was 99.9%, and the reaction was stopped.
[0060] Add 246.38g (1mol) 2-(3,4-epoxycyclohexyl)ethyltrimethoxysilane dropwise into the constant pressure dropping funnel, slowly add dropwise to the above reaction system, after 1 hour, dr...
Embodiment 2
[0065] Add 103.17g (1mol) of diethylenetriamine into a 1L four-neck round bottom flask equipped with a mechanical stirring paddle, a thermometer, a constant pressure dropping funnel, and a N 2 Air line pipe, bubbler, add 344.36g (2mol) diethyl fumarate into the constant pressure dropping funnel, pass N into the system 2 30min, replace the air in the system; slowly drop diethyl maleate into the flask under the condition of stirring at 25°C, drop it at a constant speed for 1h, control the reaction temperature at 26°C, then raise the temperature to 50°C for 6h, pass thiol- The unsaturation value measured by the iodine titration method was 0.33 mg maleic acid / g resin, indicating that the conversion rate of the maleate ester reaction was 99.9%, and the reaction was stopped.
[0066] Add 246.38g (1mol) 2-(3,4-epoxycyclohexyl)ethyltrimethoxysilane into the constant pressure dropping funnel, and slowly add it dropwise to the above reaction system, after 1 hour, the reaction The temp...
Embodiment 3
[0071] Add 116.99g (0.8mol) of triethylenetetramine into a 1L four-neck round bottom flask equipped with a mechanical stirring paddle, a thermometer, a constant pressure dropping funnel, and a N 2 Air pipe, bubbler, 275.49g (1.6mol) diethyl maleate was added to the constant pressure dropping funnel, and N was introduced into the system. 2 20min, replace the air in the system; slowly drop diethyl maleate into the flask under the condition of stirring at 25°C, drop it at a constant speed for 0.5h, control the reaction temperature at 34°C, then raise the temperature to 40°C for 5h, pass thiol - The unsaturated value measured by the iodine titration method was 0.24 mg maleic acid / g resin, indicating that the conversion rate of the maleate ester reaction was 99.9%, and the reaction was stopped.
[0072] Add 378.14 (1.6mol) γ-glycidyl propyl trimethoxysilane into the constant pressure dropping funnel, slowly add it dropwise to the above reaction system, after 2 hours, the temperatu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Epoxy value | aaaaa | aaaaa |
| Epoxy value | aaaaa | aaaaa |
| Epoxy value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com