Reduced iron production method using electrowinning method, and reduced iron produced thereby
A technology of electrolytic deposition and iron reduction, applied in the electrolysis process, electrolysis components, electrodes, etc., can solve the problem of difficulty in obtaining pure iron, and achieve the effect of high effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0028] Hereinafter, preferred embodiments of the present invention will be described in detail with reference to the accompanying drawings.
[0029] Advantages and features of the present invention, and a method for realizing them can be clearly understood with reference to the accompanying drawings and detailed embodiments described later.
[0030] However, the present invention is not limited to the embodiments disclosed below, and can be implemented in various ways that are different from each other. This embodiment only completes the disclosure of the present invention, and is intended to be complete for those of ordinary skill in the art to which the present invention belongs. The scope of the present invention is provided for an understanding of the scope of the invention, and the present invention is only defined by the scope of the claimed scope of the invention.
[0031] In addition, in the process of describing the present invention, when it is judged that related kn...
Embodiment 1
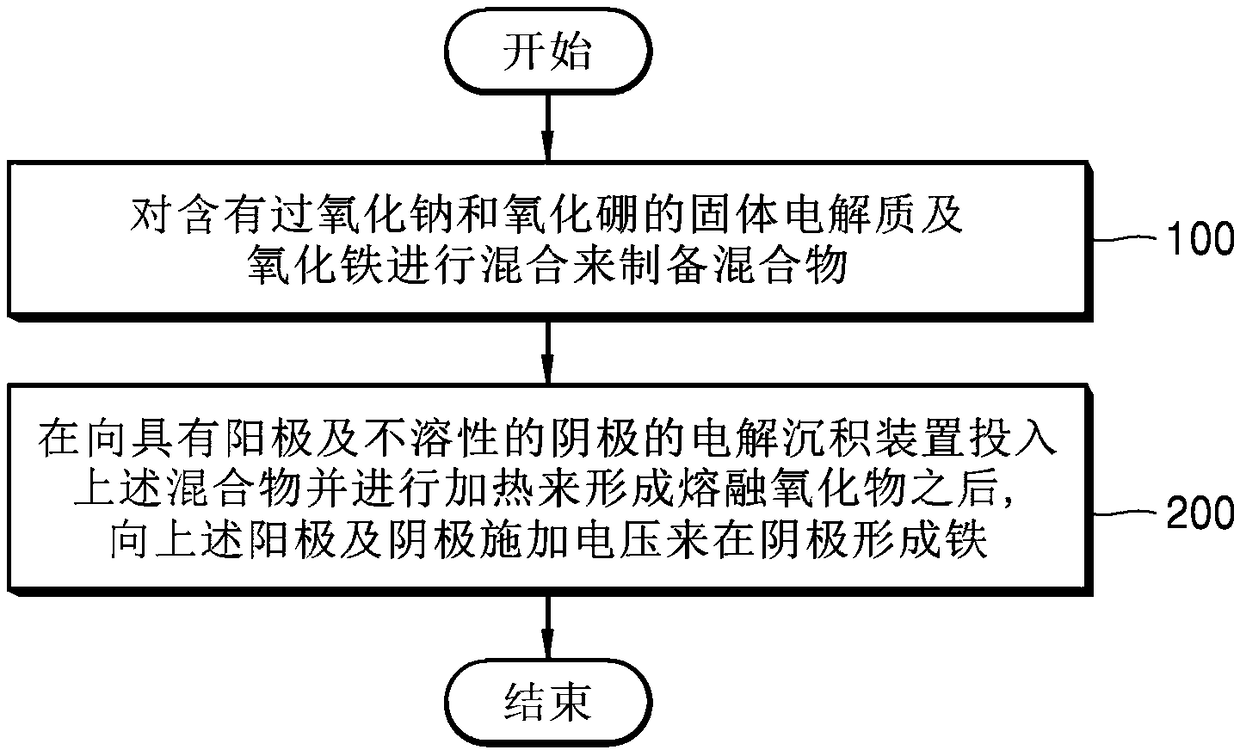
[0071] Utilize electrolytic deposition method to prepare reduced iron
[0072] First, sodium peroxide and boron oxide, which are oxides of Group I elements, are mixed to prepare a solid electrolyte. Iron oxide was mixed with the above solid electrolyte, pulverized and stirred using a ball mill to prepare a mixture.
[0073] The eutectic point was determined from a precalculated phase diagram of the ternary system.
[0074] In this case, the mixture includes 60% by weight of boron oxide, 30% by weight of sodium peroxide and 10% by weight of iron oxide.
[0075] After the above mixture is put into the electrolytic deposition device, and heated in a crucible at a temperature of 1000 ° C to prepare the above mixture into a molten oxide, the voltage difference between the anode and the cathode of the electrolytic deposition device reaches 1.5V and 2.5V mode regulation voltage to apply for 3 hours.
[0076] Species produced at the cathode as a result of electrowinning were analy...
experiment example 1
[0077] Confirmation of Reduction Reaction
[0078] In a solid electrolyte containing sodium peroxide and boron oxide, an experiment was conducted to see whether the reduction reaction in the electrowinning process can be carried out with iron oxide, which is relatively easy to reduce.
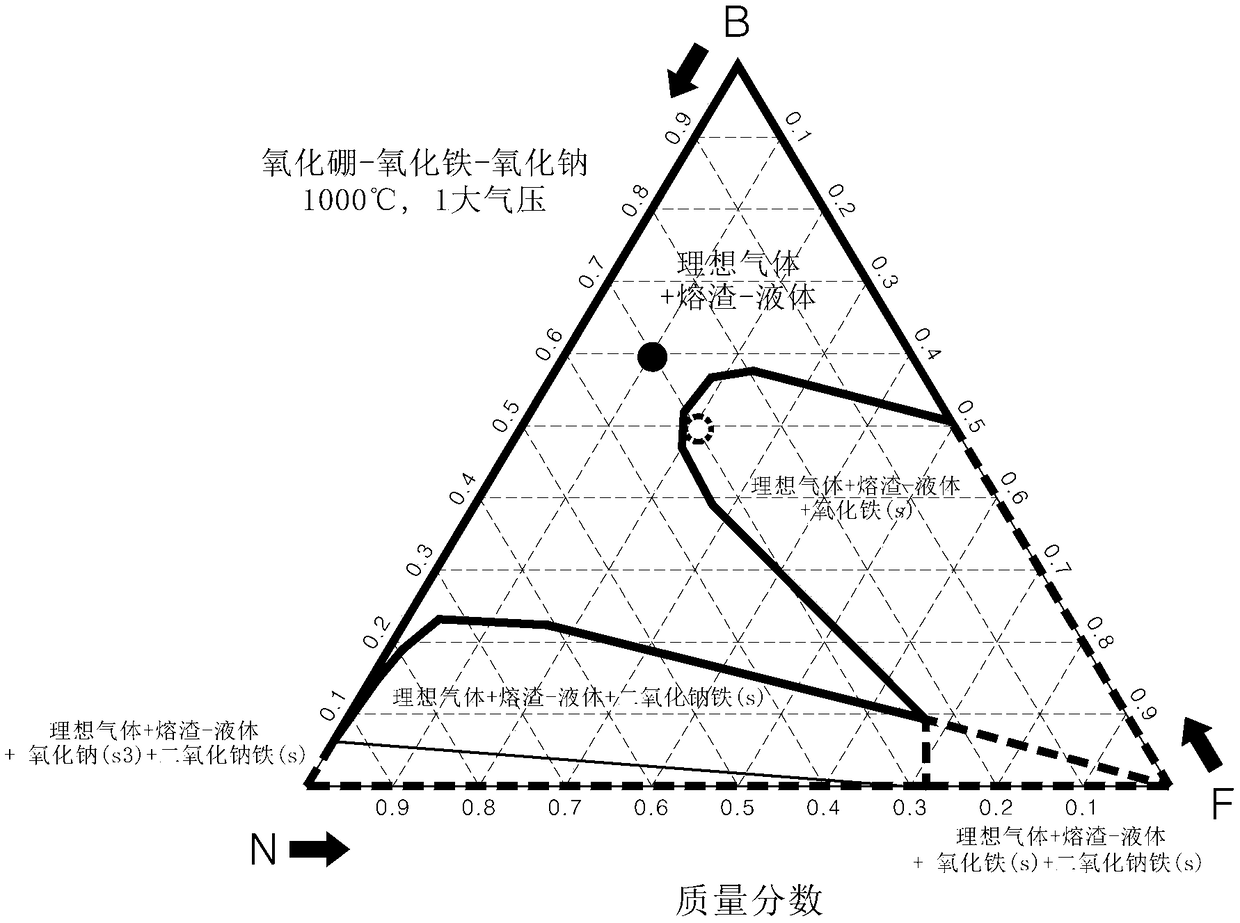
[0079] First, use the FACTSAGE program to calculate the phase diagram of the ternary system at 1000°C and 1 atm and confirm the eutectic point.
[0080] figure 2 It is the phase diagram of the ternary system of sodium peroxide, boron oxide and iron oxide calculated by the FACTSAGE program (B 2 o 3 -Na 2 o 2 -Fe 2 o 3 ).
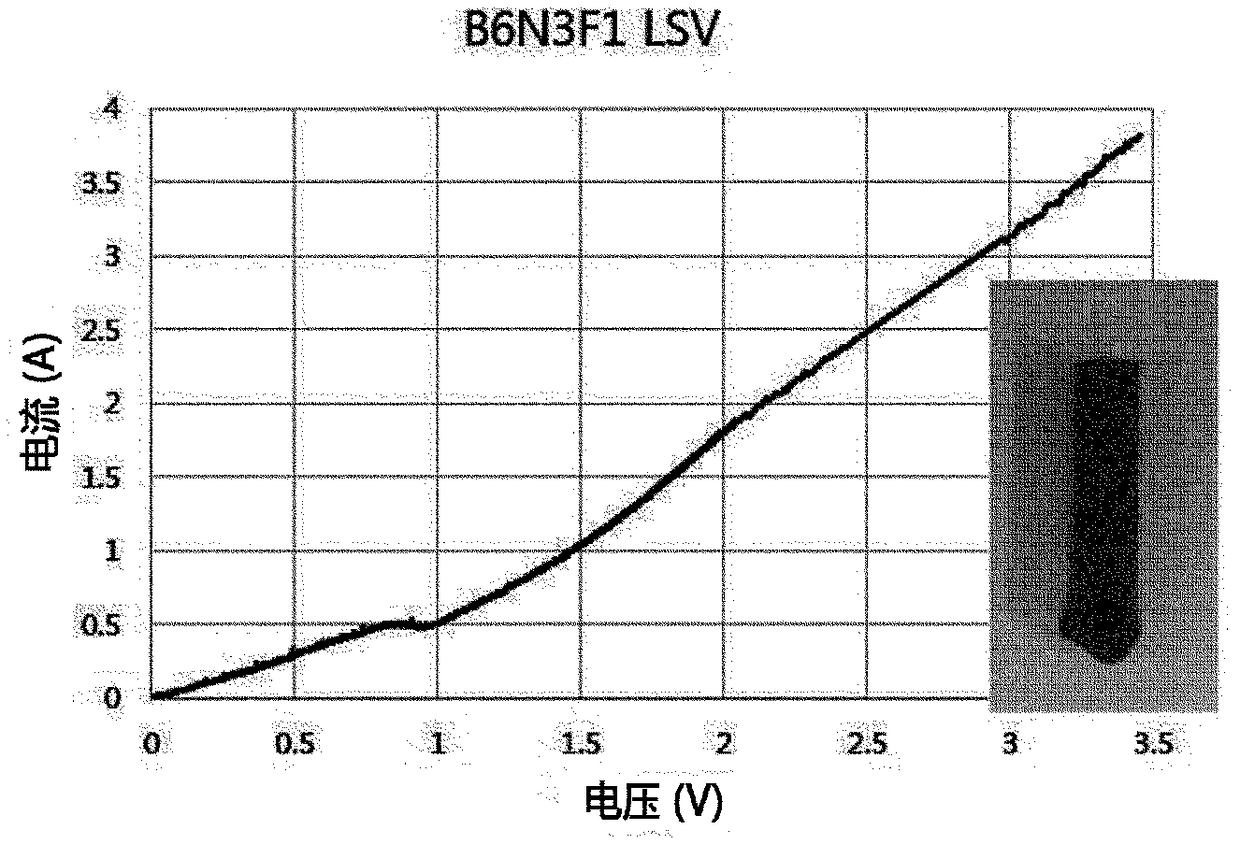
[0081] refer to figure 2 , set the combination in the process area marked with a red dot as B:N:F to 6:3:1, and denote it as B 6 N 3 f 1 , set the combination of process areas marked with black dots as B:N:F to 5:3:2, and denote it as B 6 N 3 f 2 .
[0082] When the above two combinations were heated at 1000° C. for 1 hour to confirm the eutectic point, it wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com