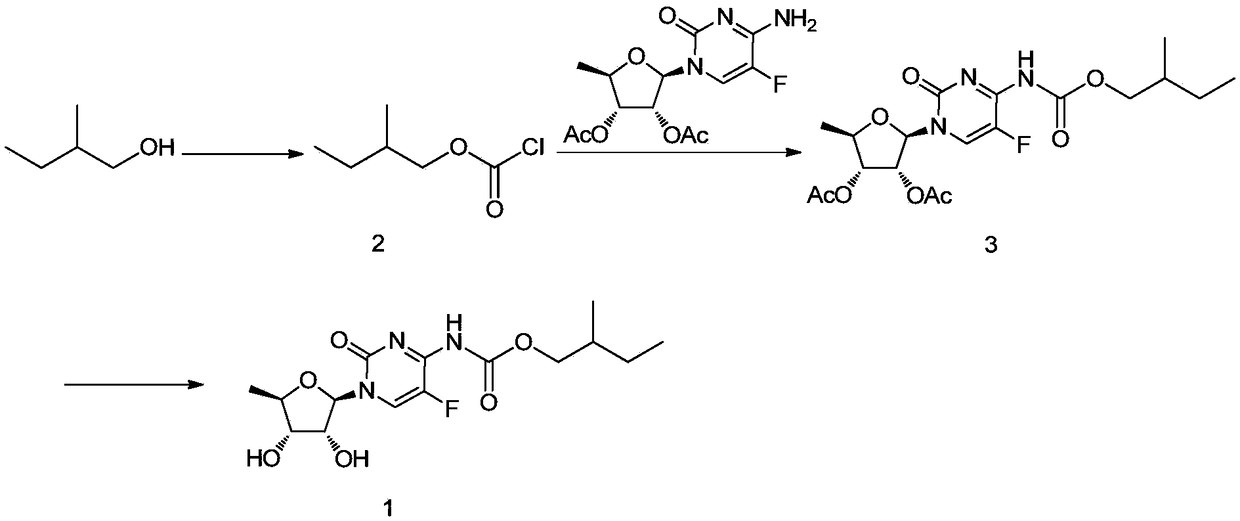
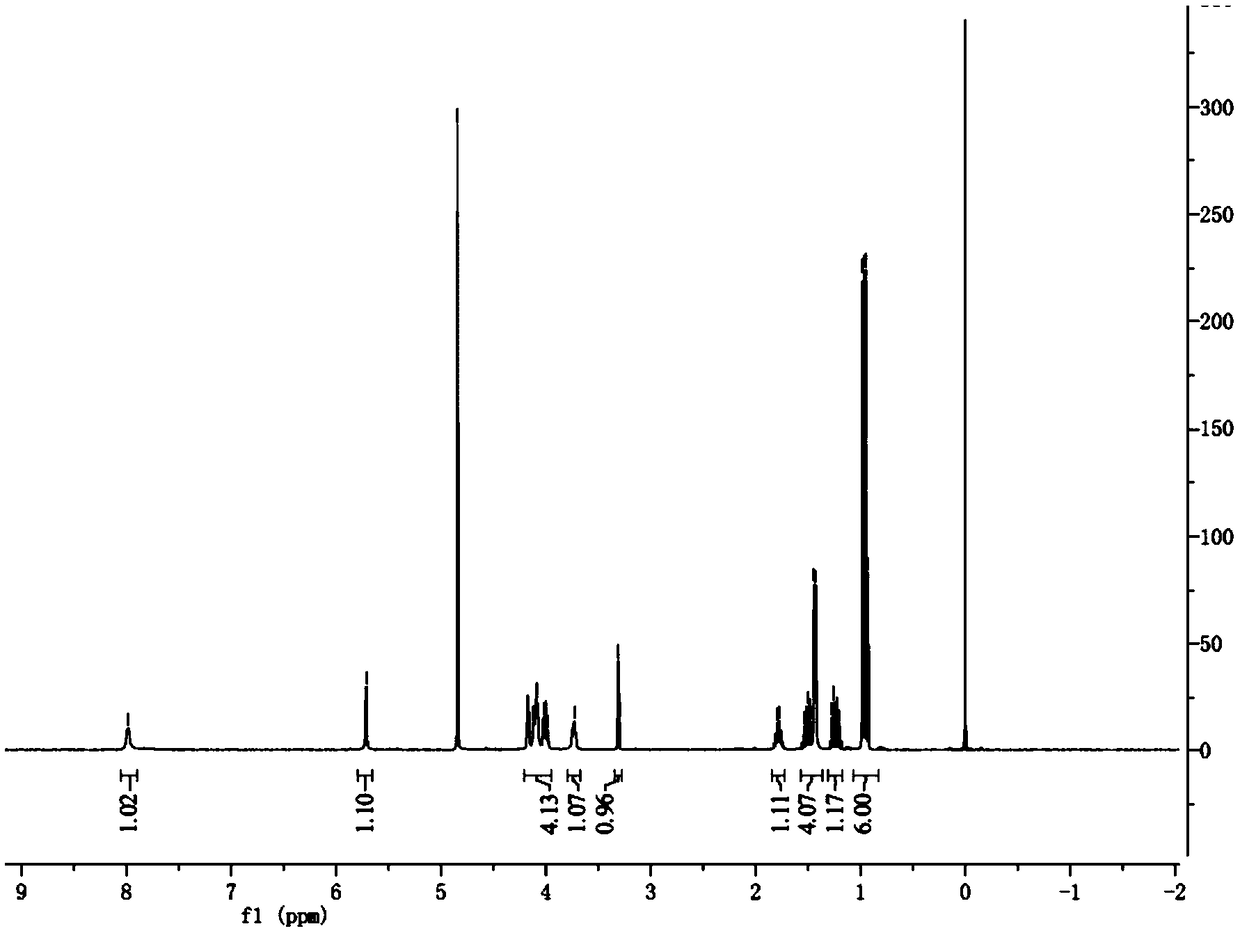
Preparation method of capecitabine impurity F
A capecitabine and impurity technology, applied in the field of organic synthesis, can solve problems such as unpublished synthetic routes, and achieve the effects of promoting acylation and simplifying process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] The preparation method of the capecitabine impurity F of the present invention comprises the following steps: (a) adding 2-methyl-1-butanol, tetrahydrofuran, triethylamine, nano-titanium dioxide and nano-ferric oxide into a reaction vessel, After nitrogen replacement, the temperature is lowered to ≤0°C, a tetrahydrofuran solution of triphosgene is added dropwise, and the reaction is carried out under the irradiation of ultraviolet light; the filtrate is filtered and spin-dried to obtain the first mixture; the nano-titanium dioxide, nano-ferric oxide and triphosgene The mass ratio is 5~10:5~10:100; (b) Add potassium carbonate, 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine to another reaction vessel After replacing with acetone and nitrogen, drop the acetone solution of the first mixture to react, and filter to obtain the reaction solution of the second mixture; (c) cool the reaction solution of the second mixture to ≤-10°C, adjust the pH to alkaline to Carry out the reacti...
Embodiment 1
[0020] The present embodiment provides a kind of preparation method of capecitabine impurity F, such as figure 1 As shown, it includes the following steps:
[0021] (a) Add 2-methyl-1-butanol (CAS: 137-32-6) (7.5g, 1.0eq), tetrahydrofuran (40 mL), triethylamine (8.6g, 1.0 eq), nano-titanium dioxide (anatase type, 2.5g) and nano-ferric oxide (2.5g), nitrogen replacement 3 times, cooling to 0 ° C, dropwise adding triphosgene (25.3g, 1.0eq) in tetrahydrofuran (40 mL), after the dropwise addition was completed, it was raised to room temperature and reacted for 0.5 hours; the reaction solution was filtered, the filter cake was washed twice with tetrahydrofuran, and the filtrate was spin-dried to obtain the first mixture (directly dropped into the first step reaction, that is, compound 2);
[0022] (b) Add potassium carbonate (23.5g, 2.0eq), 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine (CAS: 161599-46- 8, 22g, 0.8eq) and acetone (100ml), replace with nitrogen for 3 times, and slowl...
Embodiment 2
[0025] This example provides a method for preparing capecitabine impurity F, which is basically the same as that in Example 1, except that in step (a), anatase-type nano-titanium dioxide (anatase-type, 1.3 g) and nanometer ferric oxide (2.6g), then warmed up to room temperature and reacted for 1 hour; finally, 11.5g of compound 1 was obtained by column chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com