Preparation method of N-vinyl-1,2,3-triazole compound
A compound, vinyl technology, applied in the field of preparation of N-vinyl-1,2,3-triazole compounds, can solve problems such as damage, achieve easy separation and purification, excellent substrate universality, good Yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] A preparation method of N-vinyl-1,2,3-triazole compounds, the reaction process is as shown in formula (3):
[0054]
[0055] The reaction specifically includes the following steps:
[0056] (1) Compound 1a, namely phenylacetylene (34uL, 0.3mmol), compound 2 (82mg, 0.2mmol), NaBH 4 (3.8mg, 0.4mmol), join in 1mL acetonitrile and 1mL triethylamine, then add catalyst Pd(PPh 3 ) 4 (4.7mg, 0.004mmol) and CuI (1.5mg, 0.008mmol), under argon protection, heated and stirred at 60°C for 4h, TLC detected that the substrate disappeared, and the reaction was over; the liquid;
[0057] (2) the viscous liquid obtained in step (1) is subjected to silica gel column chromatography (eluent is V 石油醚 :V 乙酸乙酯 =5:1) to obtain compound 3a with a yield as high as 91%.
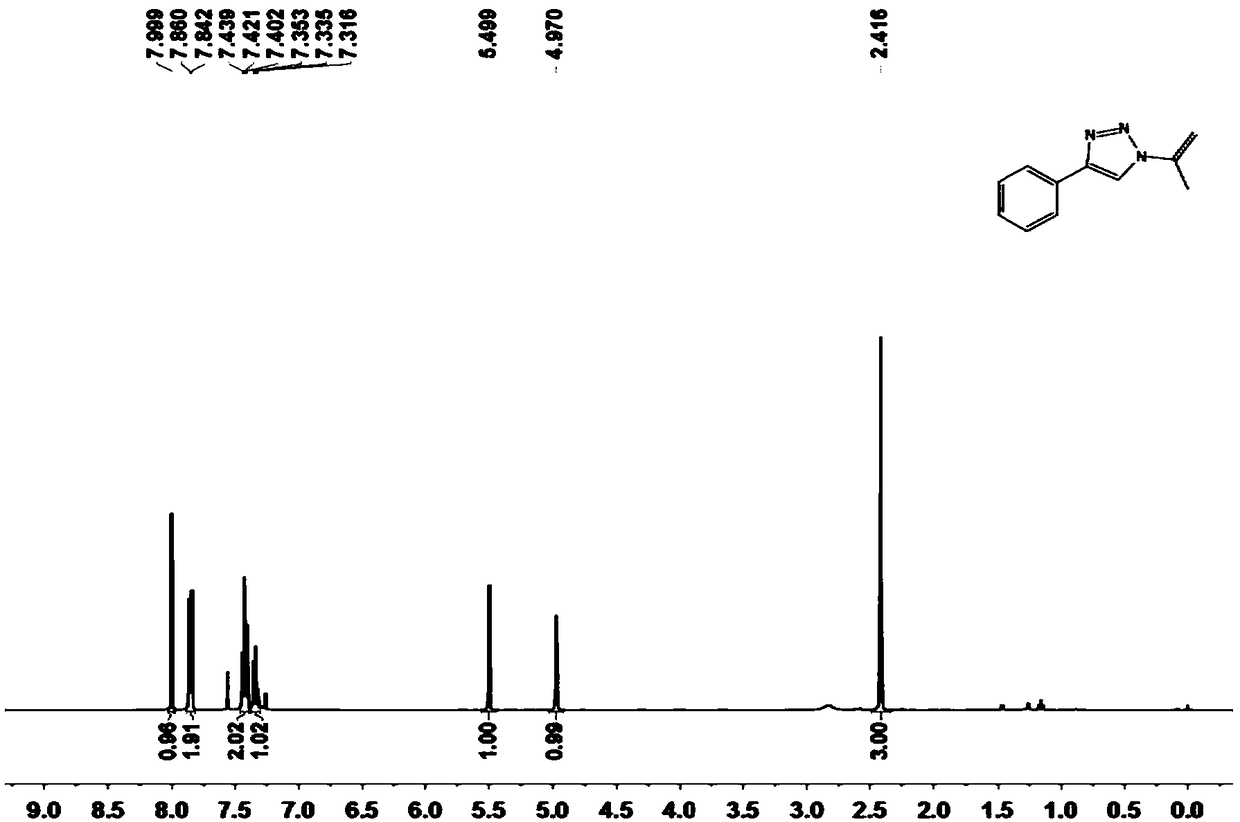
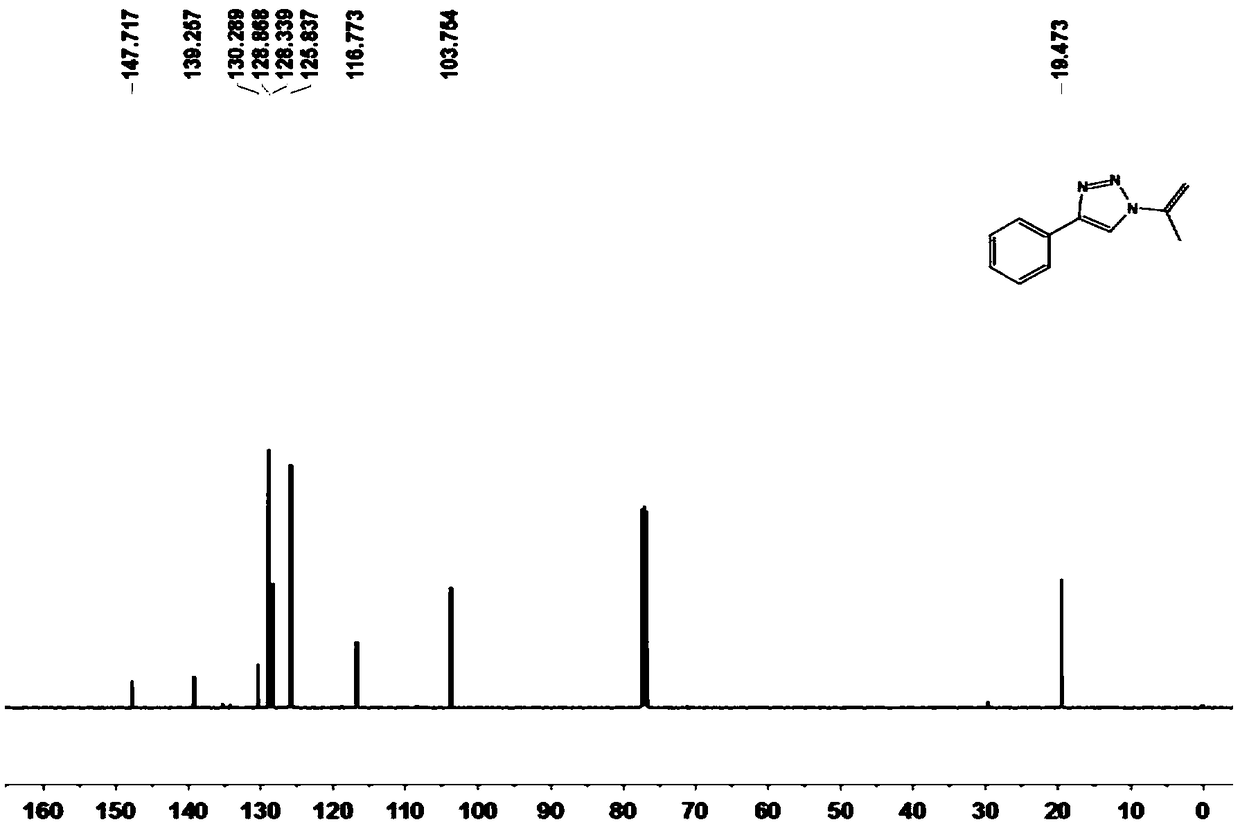
[0058] Compound 3a prepared in the present embodiment 1 H-NMR and 13 The nuclear magnetic resonance spectra of C NMR are as follows figure 1 and 2 As shown, the result is:
[0059] 1 H NMR (400MHz, CDCl 3 )δ8.00(s...
Embodiment 2
[0066] A preparation method of N-vinyl-1,2,3-triazole compounds, the reaction process is as formula (3) in Example 1; the difference is:
[0067] (1) Compound 1a is phenylacetylene (0.2mmol), compound 2 (82mg, 0.2mmol), NaBH 4 (3.8mg, 0.4mmol), was added to 1mL water and 0.8mL triethylamine, then added catalyst Pd(PPh 3 ) 4 (0.008mmol) and CuI (0.004mmol), under argon protection, heated and stirred at 80°C for 4h, until the TLC detection substrate disappeared, and the reaction ended; the reaction solution was distilled under reduced pressure to remove the organic solvent to obtain a viscous liquid;
[0068] (2) the viscous liquid obtained in step (1) is subjected to silica gel column chromatography (eluent is V 石油醚 :V 乙酸乙酯 =5:1) Compound 3a was obtained with a yield of 65%.
Embodiment 3
[0070] A preparation method of N-vinyl-1,2,3-triazole compounds, the reaction process is as formula (3) in Example 1; the difference is:
[0071] (1) Compound 1a, namely phenylacetylene (34uL, 0.3mmol), compound 2 (82mg, 0.2mmol), NaBH 4 (3.8mg, 0.4mmol), was added in 1mL methanol and 1mL triethylamine, then added catalyst Pd(PPh 3 ) 4 (0.004mmol) and CuCl 2 (1.4mg, 0.008mmol), protected by argon, heated and stirred at 60°C for 4h, until the substrate disappeared as detected by TLC, and the reaction was completed; the reaction solution was distilled under reduced pressure to remove the organic solvent to obtain a viscous liquid;
[0072] (2) the viscous liquid obtained in step (1) is subjected to silica gel column chromatography (eluent is V 石油醚 :V 乙酸乙酯 =5:1) to obtain compound 3a with a yield as high as 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com