Hypotensive pharmaceutical composition
A composition and drug technology, applied in directions such as drug combinations, active ingredients of hydroxyl compounds, pharmaceutical formulations, etc., can solve the problems of large differences in release rates, unfavorable medication compliance, and increase the number of medication, so as to ensure the antihypertensive efficacy, The effect of promoting microcirculation and delaying the release rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] Preparation:
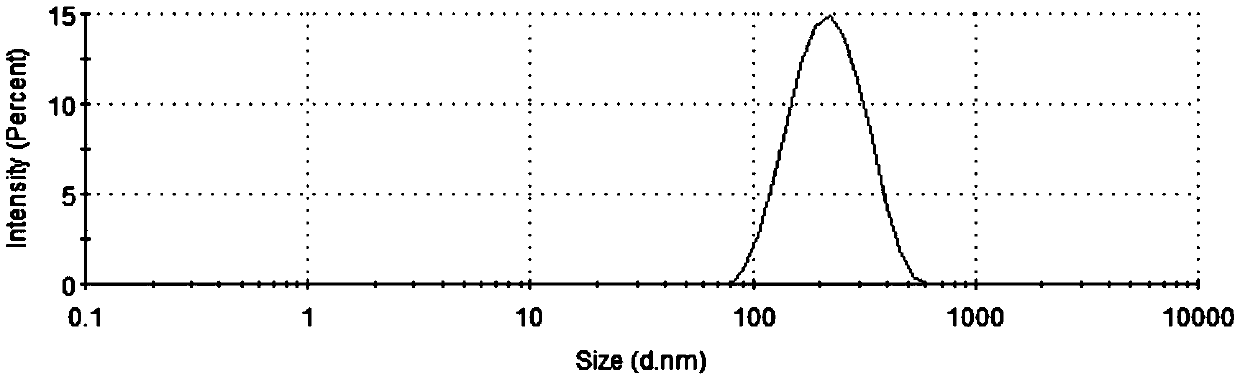
[0031] 1) Preparation of daidzein and honokiol liposomes: Weigh soybean phosphatidylcholine, cholesterol, polyethylene glycol-distearoylphosphatidylethanolamine, daidzein and honokiol, and vortex to make It is dissolved in a mixed solvent of chloroform and methanol (1:4, V / V), and then the liposome suspension is prepared according to the thin film ultrasonic dispersion method: firstly, the mixed solution is evaporated under reduced pressure at 40°C to remove the organic solvent to the inner wall of the container Form a layer of liposome film, then add ultrapure water to make the solid content 0.15%, stir the hydrated liposome film at 60°C, then ultrasonically break and homogenize the liposome in an ice bath, and finally filter through 0.22 μm The membrane is sized to prepare the daidzein and honokiol long-circulating liposome suspension.
[0032] The entrapment efficiency of daidzein and honokiol encapsulation liposomes was measured by dialys...
Embodiment 2
[0053] A kind of antihypertensive pharmaceutical composition, it is made up of the composition of following weight ratio:
[0054]
[0055] The preparation method comprises the following steps:
[0056] 1) Dissolve daidzein, honokiol, soybean phosphatidylcholine, cholesterol, polyethylene glycol-distearoylphosphatidylethanolamine in a mixed solvent of chloroform and methanol (1:4, V / V), Then make a liposome suspension with a solid content of 0.3% according to the thin-film ultrasonic dispersion method: first remove the organic solvent by rotary evaporation under reduced pressure at 40 ° C of the mixed solution to form a liposome film on the inner wall of the container, then add ultrapure water to make The solid content is 0.3%, stirring the hydrated liposome film at 60°C, then ultrasonically crushing and homogenizing the liposome in an ice bath, and finally passing through a 0.22 μm filter membrane for granulation, so as to obtain daidzein, and thick Parkinol long-circulat...
Embodiment 3
[0063] A kind of antihypertensive pharmaceutical composition, it is made up of the composition of following weight ratio:
[0064]
[0065]
[0066] The encapsulation efficiency of daidzein was measured to be 57.8%, and the encapsulation efficiency of honokiol was 95.6%.
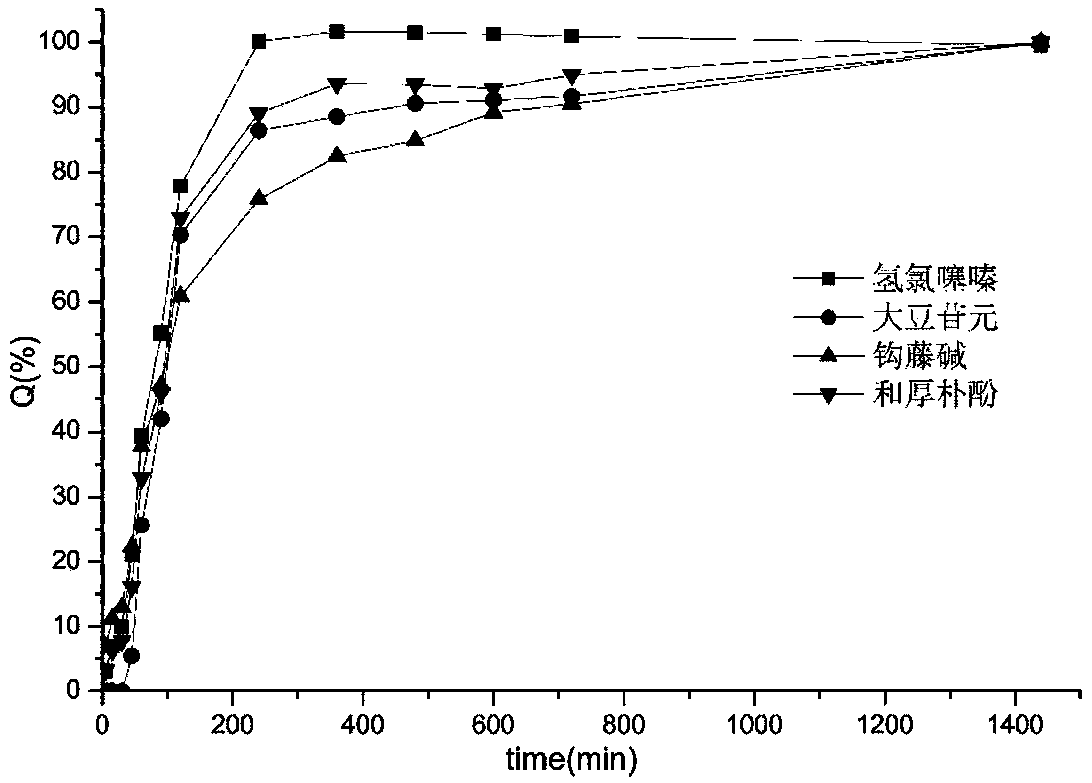
[0067] The components of the tablet are basically completely released within 8 hours, and the similar factors of synchronous release are shown in Table 3.
[0068] Each component of table 3 embodiment 3 liposome tablet releases similar factor synchronously
[0069] substance
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com