A kind of citicoline sodium injection and preparation method thereof
A technology for citicoline sodium and injection, which is applied in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problem of reducing the safety and reliability of citicoline sodium injection. It has the advantages of good market application prospect, simple preparation method and improved stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The present invention also provides a kind of preparation method of described Citicoline Sodium Injection, comprising the following steps:
[0030] (1) Dissolve citicoline sodium, disodium cytidine triphosphate and ginsenoside Rg1 in 50-60 vol% water for injection, then adjust the pH to 5.7-6.3 with a pH regulator, and then cool down to 3-5 ℃, and pass CO 2 20~60min, obtain medicinal liquid A;
[0031] (2) adding medicinal charcoal to the medicinal solution A, stirring and adsorbing for 15-45 min, decarbonizing and filtering, then adding glutathione, a stabilizer, an isotonicity agent and the remaining 40-50 vol% water for injection to obtain liquid B;
[0032] (3) filtering medicinal liquid B with a microporous membrane of 0.2~0.5 μm, then feeding nitrogen to nitrogen saturation, then filling and sealing in nitrogen flow to obtain semi-finished product of Citicoline Sodium Injection;
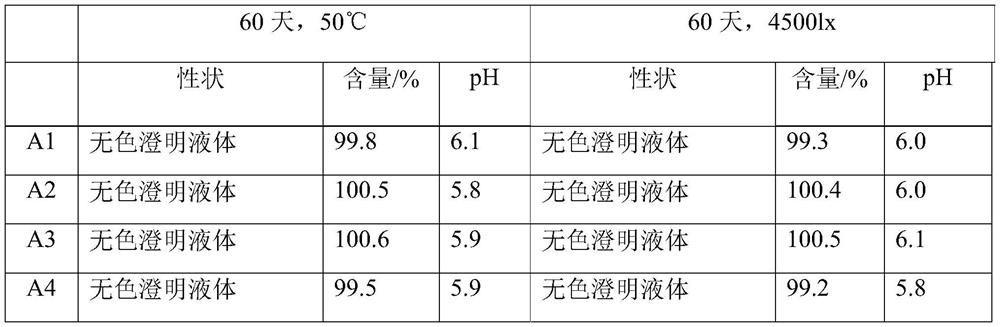
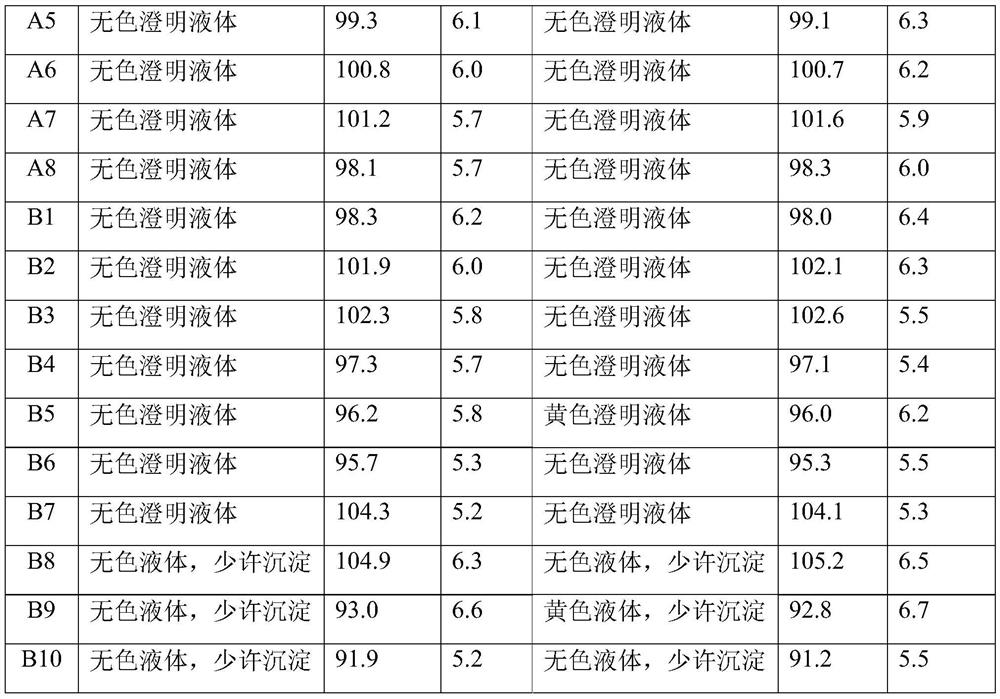
[0033] (4) The semi-finished product of Citicoline Sodium Injection is sterilize...
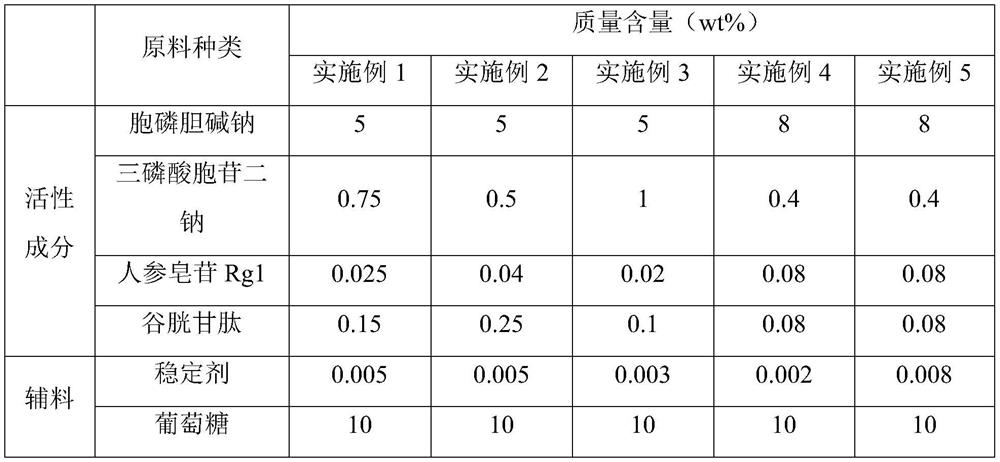
Embodiment 1
[0041] A kind of Citicoline Sodium Injection, its raw material composition is as shown in Table 1, and concrete preparation method is as follows:
[0042] (1) 60g citicoline sodium was mixed in 200mL ethanol to obtain a suspension, the suspension was filtered to obtain a filtrate and a filter cake, then the filter cake was dissolved in 300mL water to obtain a mixed solution, then the mixed solution was Pre-freeze at -32°C and 2Pa for 1.5h, then heat up to 35°C and keep for 1h to obtain the refined product of citicoline sodium;
[0043] Dissolve 50g of citicoline sodium refined product, 7.5g of cytidine triphosphate disodium, 0.25g of ginsenoside Rg1 in 462.38mL of water for injection, and then use a pH adjuster (consisting of 0.1mol / L citric acid and 0.1mol / L sodium citrate according to the volume ratio of 1:2.64) to adjust the pH value to 5.7-6.3, then cool down to 3-5 °C, and pass CO 2 30min, obtain medicinal liquid A;
[0044] (2) Add 1 g of medicinal charcoal to the me...
Embodiment 2
[0048] A kind of Citicoline Sodium Injection, its raw material composition is as shown in Table 1, and concrete preparation method is as follows:
[0049] (1) 60g citicoline sodium was mixed in 200mL ethanol to obtain a suspension, the suspension was filtered to obtain a filtrate and a filter cake, then the filter cake was dissolved in 300mL water to obtain a mixed solution, then the mixed solution was Pre-freeze at -32°C and 2Pa for 1.5h, then heat up to 35°C and keep for 1h to obtain the refined product of citicoline sodium;
[0050] Dissolve 50g of citicoline sodium refined product, 5g of cytidine triphosphate disodium, 0.4g of ginsenoside Rg1 in 463.13mL of water for injection, and then use a pH adjuster (consisting of 0.1mol / L citric acid and 0.1mol / L). L sodium citrate is composed according to the volume ratio of 1:2.64) to adjust the pH value to 5.7 to 6.3, then cool down to 3 to 5 ° C, and pass CO 2 45min, obtain medicinal liquid A;
[0051] (2) Add 1 g of medicinal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com