Polypeptide as well as preparation method and application thereof in inhibition of HIV
A lentivirus and amino acid technology, applied in the field of biomedicine, can solve the problems of being unable to clear HIV latent pools to cure AIDS, unable to kill HIV-infected cells, and achieve the effect of less drug-resistant strains
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
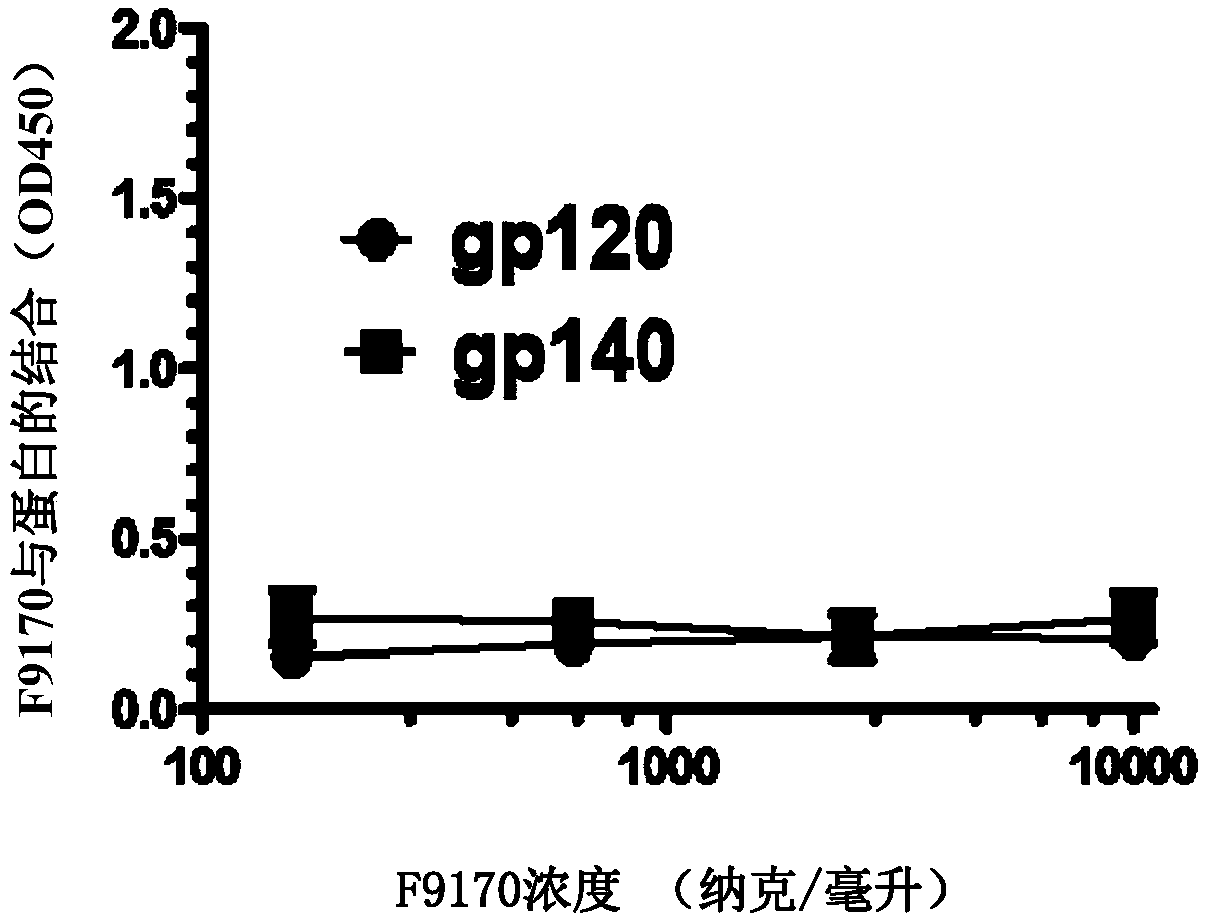
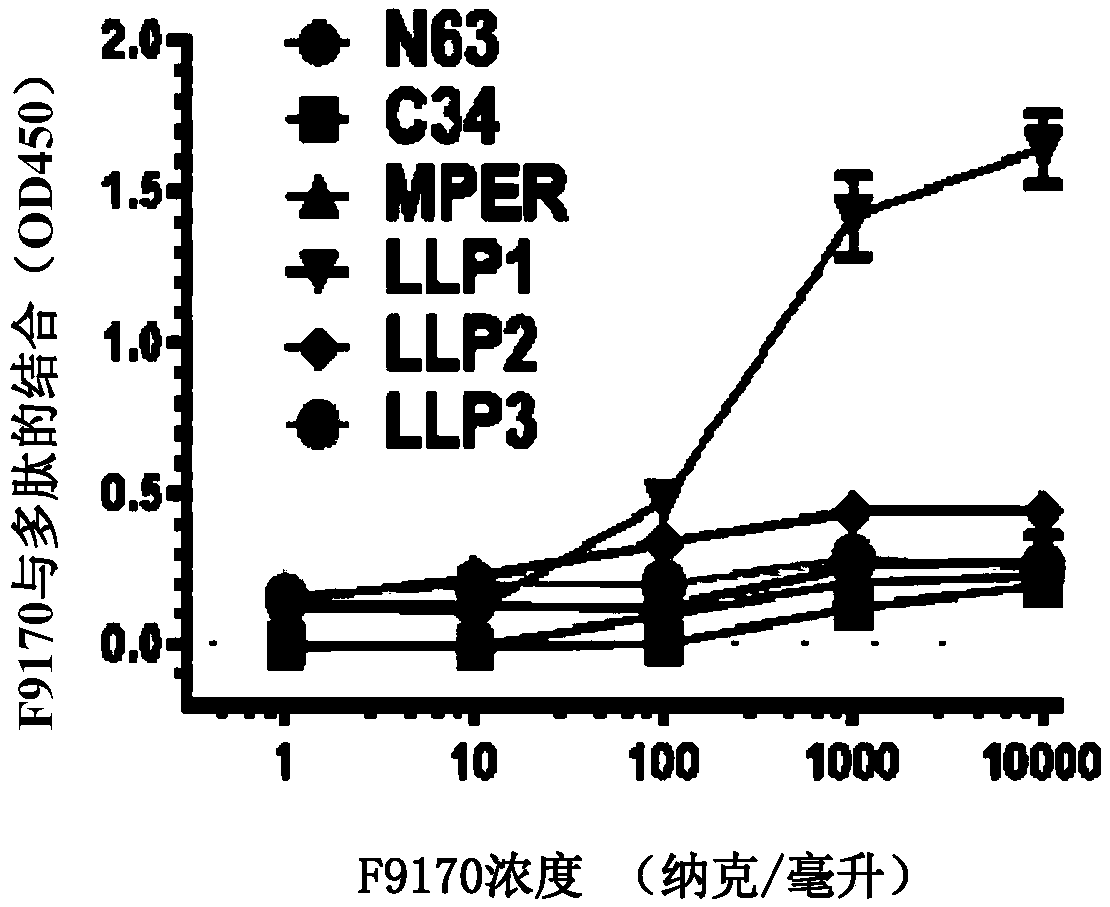
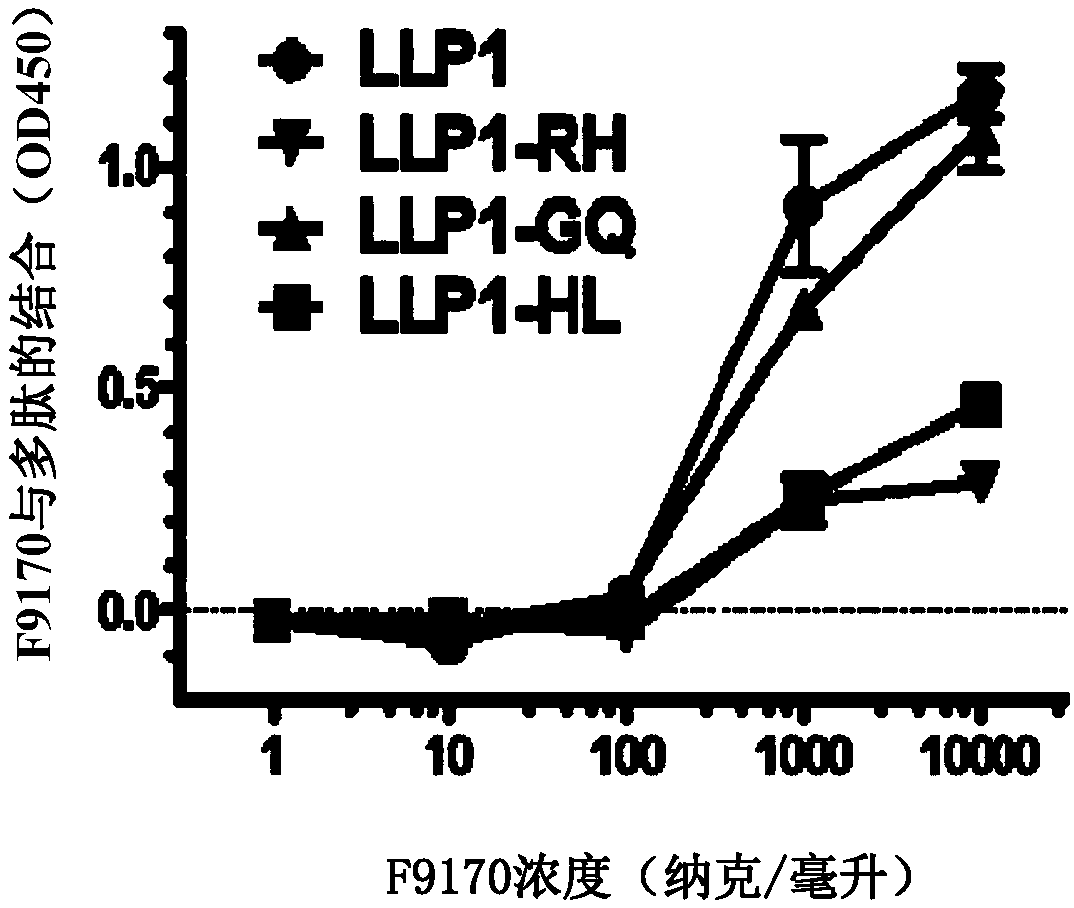
[0096] Embodiment 1: Binding experiment of the polypeptide of the present invention
[0097] 1.1 Experimental materials and methods
[0098]Based on enzyme-linked immunosorbent assay (ELISA), it was detected whether the F9170 polypeptide (SEQ ID NO: 2) (synthesized by Synpeptide Co., Ltd.) could interact with the following HIV envelope protein-derived proteins or polypeptides (all Synpeptide Co., Ltd. Synthetic) combination: gp120 (SEQ ID NO:19): HIV envelope protein gp120 subunit; gp140 (SEQ ID NO:20): HIV envelope protein removes the transmembrane domain and intracellular sequence part; N63 (SEQ ID NO:21): NHR domain on HIV envelope protein subunit gp41; C34 (SEQ ID NO:22): CHR domain on HIV envelope protein subunit gp41; MPER (SEQ ID NO:23): HIV envelope Proximal membrane domain on protein subunit gp41; LLP1(SEQ ID NO:24) / LLP2(SEQ ID NO:25) / LLP3(SEQ ID NO:26): HIV envelope protein subunit gp41 intracellular segment LLP1 / LLP2 / LLP3 domain; LLP1-RH (SEQ ID NO: 27): 828-842 ...
Embodiment 2
[0102] Embodiment 2: Polypeptide of the present invention inactivates HIV virus
[0103] 2.1 Experimental materials and methods
[0104] Detection of F9170 polypeptide (SEQ ID NO:2), F9170 scrambled polypeptide (F9-scr) (SEQ ID NO:30) and T20 (SEQ ID NO:18) (all synthesized by Synpeptide Co., Ltd.) to inactivate HIV Virus strain HIV-1IIIB, Bal, TZA68 / 125A and 92TH009 virus strain (from NIH AIDS Reagent Program, product number is 398,510,11255,1656 respectively) The method of ability is as follows: each 50 μ L above-mentioned each HIV virus (with serum-free 1640 medium (Meilunbio, product number MA0215, the 1640 medium used in all embodiments is this product) dilution, the final concentration is 200 times TCID 50 ) and 50 μL diluted (4 times) F9170 polypeptide, F9170 scrambled polypeptide and T20 (diluted with serum-free 1640 medium, initial concentration is 5 μM) and incubated at 4°C for 1 hour. Add PEG6000 (Sigma-Aldrich, Cat. No. 1546580) to make the final concentration 3%...
Embodiment 3
[0108] Example 3. F9170 specifically inhibits HIV pseudoviruses.
[0109] 3.1 Experimental materials and methods
[0110] Pseudovirus packaging: HIV envelope protein particles or MERS-CoV envelope protein particles (HIV envelope protein particles are from AIDS Reagent Program, item number is 324; MERS-CoV envelope protein particles are from cooperative research group New York Blood Center Viral Immunology research group) and HIV backbone plasmid (pNL4-3.Luc.R-E, from NIH AIDS ReagentProgram, item number: 3418) were used to package HIV and MERS-CoV pseudoviruses. Two kinds of plasmid HIV envelope protein particles or MERS-CoV envelope protein particles and HIV backbone plasmid (5 μg DNA melted in 100 μl normal saline) were simultaneously used VigoFect reagent (Vigolas Biotechnology (Beijing) Co., Ltd., catalog number T001) ( 2 μl of reagent diluted to 100 μl of saline) were transfected into 293T cells (purchased from ATCC) according to the manufacturer's instructions (200,000-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com