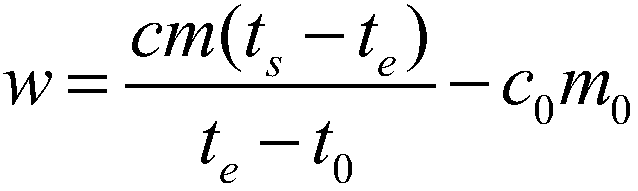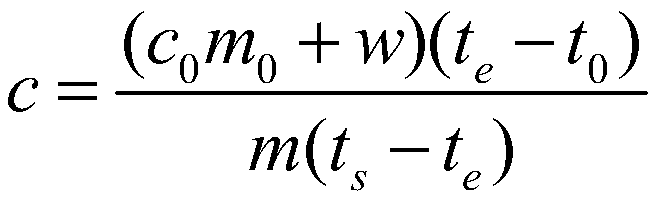Device and method for measuring specific heat capacity and phase change latent heat of substance
A technology of phase change latent heat and specific heat capacity, applied in the field of thermophysical property measurement of substances, can solve the problems of calorimetric liquid temperature drop, easy volatilization, measurement error, etc., and achieve the effect of small equipment size and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Embodiment 1: to the specific heat capacity measurement of solid red copper
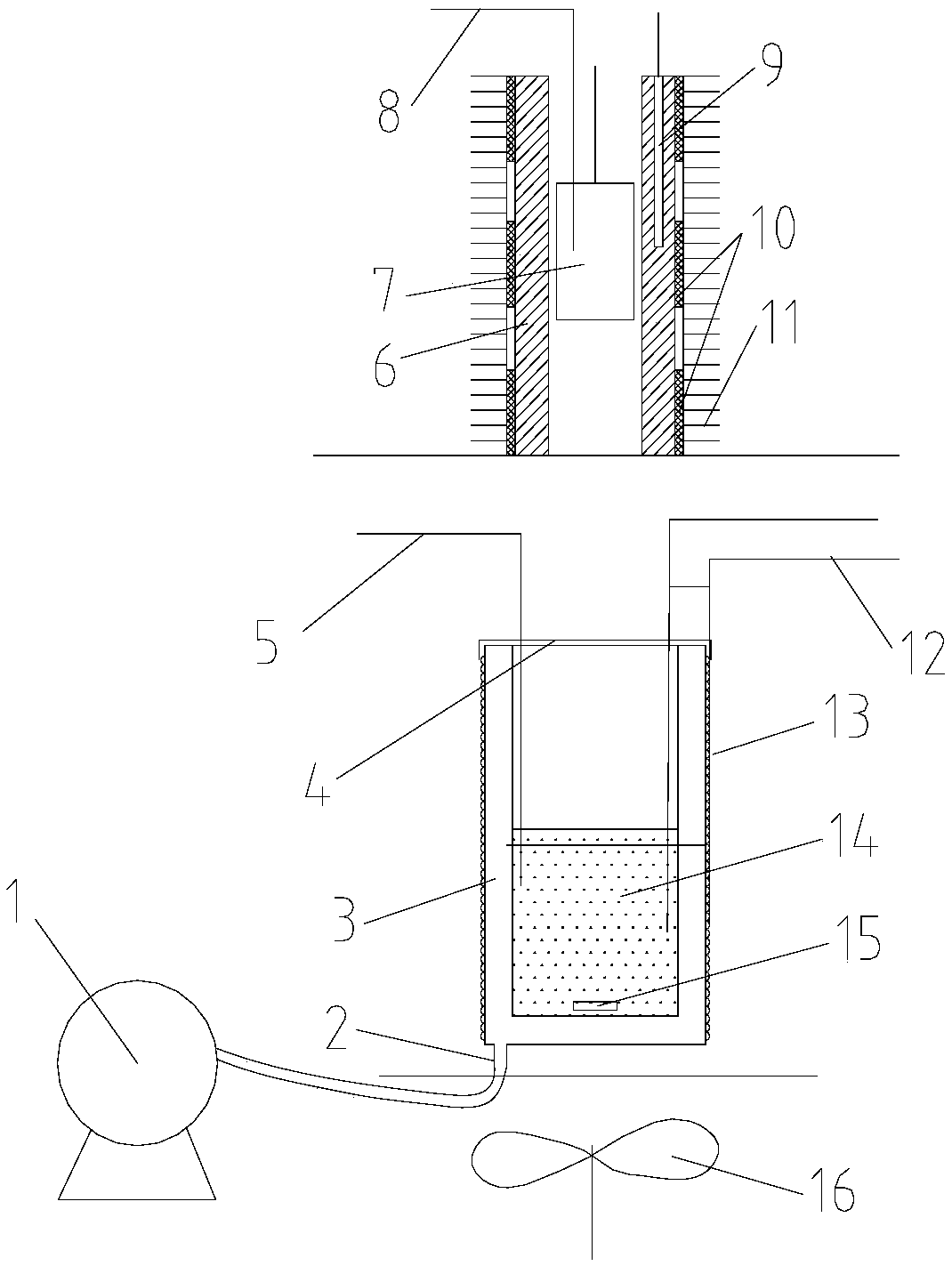
[0079] Take a copper block of a certain volume, punch a small hole in the copper block for suspension, then weigh its mass as 0.15687kg, stick the thermistor on the surface of the copper block, and hang it in the constant temperature aluminum block with a thin fishing line For temperature control, pour a certain mass of ethylene glycol into the calorimeter and keep it at a constant temperature, and use a thermistor to measure the temperature of ethylene glycol. When the temperature of the copper block and ethylene glycol no longer changes, quickly put the copper block into the ethylene glycol, when the temperature of the two thermistors no longer changes, record the equilibrium temperature at this time, according to Calibrate the heat capacity of the calorimeter obtained, and measure the specific heat capacity of the measured object. After completing an experiment, clean the copper block, rew...
Embodiment 2
[0082] Embodiment 2: to the specific heat capacity measurement of 304 stainless steel powder
[0083] Take a certain quality of 304 stainless steel powder, wrap it with a 500-mesh nylon net, embed the Pt-100 platinum resistance chip in the 304 stainless steel powder, draw it out with three 0.1mm enamelled copper wires, and hang the nylon net with a thin fishing line The temperature is controlled in a constant temperature aluminum block, a certain mass of ethylene glycol is poured into the calorimeter and kept at a constant temperature, and the temperature of the ethylene glycol is measured using a Pt-100 platinum resistance chip. When the temperature of the copper block and ethylene glycol no longer changes, quickly put the nylon mesh and 304 stainless steel powder into the ethylene glycol, when the temperature of the two Pt-100 platinum resistance chips no longer changes, record At this equilibrium temperature, the specific heat capacity of the measured object is measured acc...
Embodiment 3
[0087] Embodiment 3: the measurement of paraffin wax specific heat capacity and phase change latent heat
[0088] It is known that the starting melting temperature of a certain paraffin is 41.1°C, and the complete melting temperature is 43.9°C. Weigh different qualities of paraffin and ethylene glycol respectively, put the paraffin in an ultra-thin plastic bag, place it in a constant temperature aluminum block to control the temperature to different temperatures, and wait for the temperature of the tested sample to be constant, put it quickly After entering the ethylene glycol at constant temperature for heat exchange, after the temperature of paraffin wax and ethylene glycol no longer changes, the equilibrium temperature is measured, and the specific experimental data are shown in Table 4.
[0089] Three groups of results that table 4 embodiment 3 measures
[0090]
[0091] Using the fitting software 1stOpt to fit, the solid specific heat capacity of paraffin wax is 2663....
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com